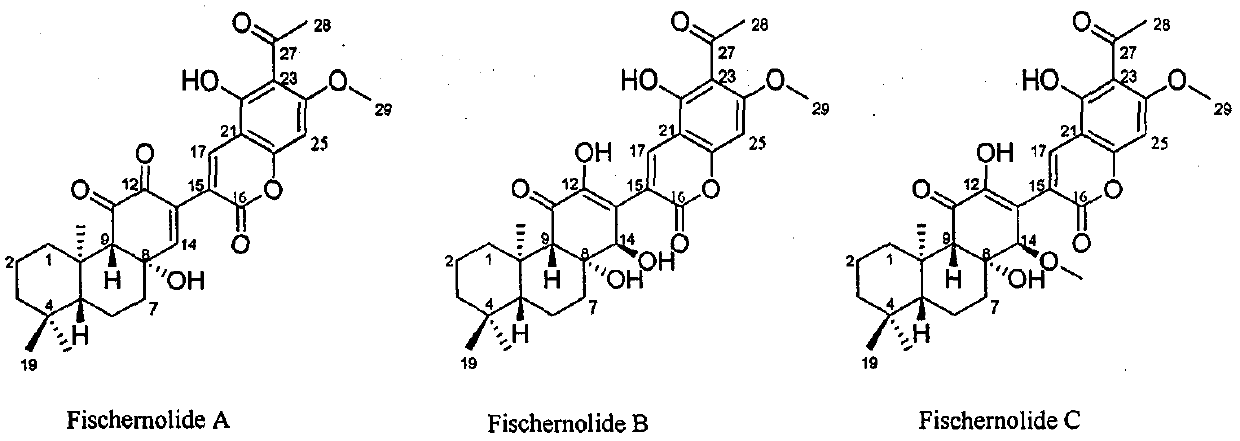
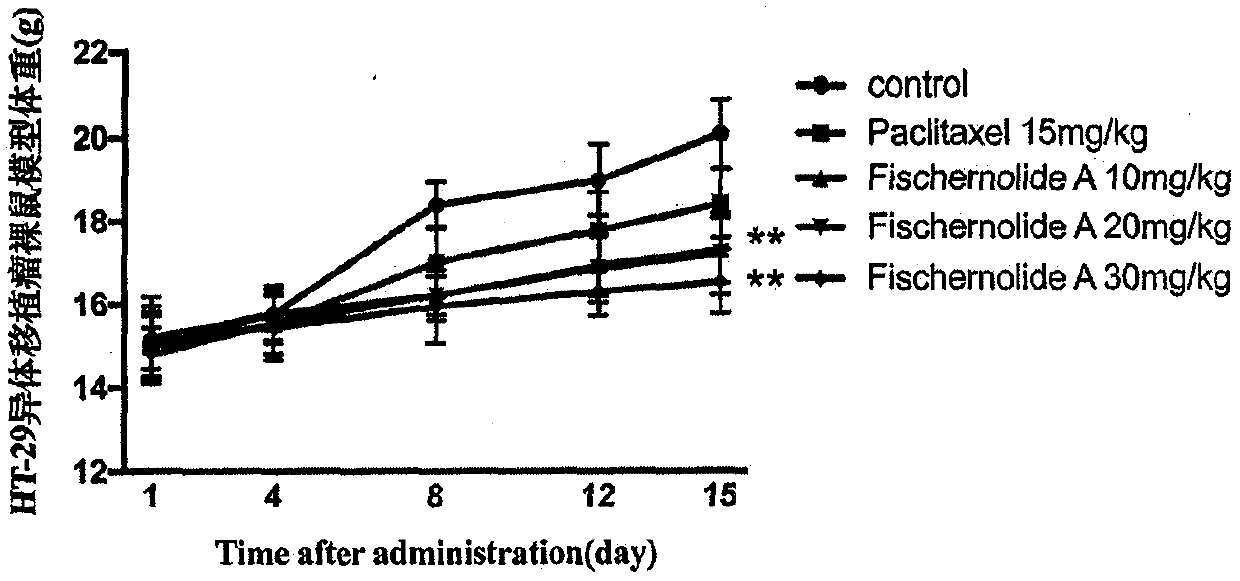
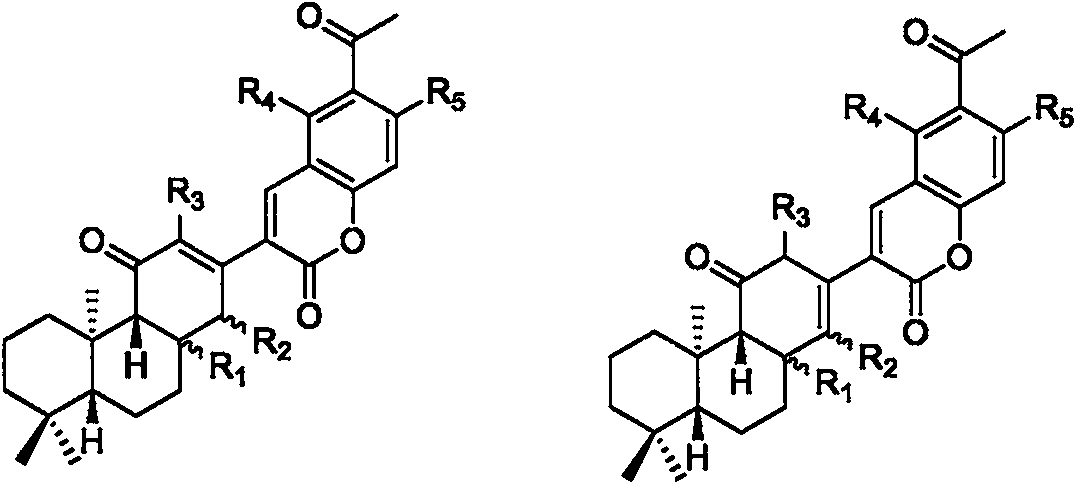
Novel skeleton meroterpenoid compound and its preparation method, pharmaceutical composition and application in anticancer medicines
A technology of compounds and compositions, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Get 10 kg of dried Euphorbia chamaejascens root, pulverize, add 10 times the amount of 95% ethanol to reflux and extract 3 times, the extraction temperature is preferably 75 ° C, extract for 2 hours each time, combine the extracts, filter, concentrate under reduced pressure, to obtain a concentrate;
[0043] (2) The concentrated solution obtained in (1) was extracted 3 times with ethyl acetate, combined, and the organic solvent was recovered to obtain extract I;
[0044] (3) The extract I was subjected to silica gel column chromatography, and gradient elution was carried out by using an organic reagent, using petroleum ether-ethyl acetate gradient elution (0:1-1:0), and using a silica gel thin-layer plate for detection, Combined into 7 (A-G) fractions; then select E to carry out reversed-phase silica gel column chromatography, methanol-water gradient elution (20:80-100:0), and a total of 5 fractions ( E1-E5) fractions; select EE5 to carry out column chromatography ...
Embodiment 2
[0047] (1) Take 20kg of dry Euphorbia chamaejasmeum roots, crush them, add 10 times the amount of 80% ethanol to reflux and extract for 3 times, the extraction temperature is preferably 70°C, extract for 2.5 hours each time, combine the extracts, filter, concentrate under reduced pressure, to obtain a concentrate;
[0048] (2) The concentrated solution obtained in (1) was extracted 3 times with ethyl acetate, combined, and the organic solvent was recovered to obtain extract I;
[0049] (3) Extract I was subjected to silica gel column chromatography, gradient elution was carried out using organic reagents, petroleum ether-acetone gradient elution (0: 1-1: 0), and detection by silica gel thin-layer plate was combined into 8 (A-H) fractions; then choose F to carry out reverse-phase silica gel column chromatography, methanol-water gradient elution (30:70-100:0), and obtain 7 (F1- F7) Fractions; select F5 to carry out column chromatography through Sephadex LH-20 dextran gel, chlor...
Embodiment 3
[0052] (1) Take 5 kg of dry Euphorbia chamaejasba root, pulverize it, add 12 times the amount of 85% ethanol to reflux and extract 4 times, the extraction temperature is preferably 80 ° C, extract for 2 hours each time, combine the extracts, filter, and concentrate under reduced pressure to obtain Concentrate;
[0053] (2) The concentrated solution obtained in (1) was extracted 3 times with ethyl acetate, combined, and the organic solvent was recovered to obtain extract I;
[0054] (3) Extract I was subjected to silica gel column chromatography, gradient elution was carried out with organic reagents, gradient elution with chloroform-methanol (0: 100-1: 0), and detection by silica gel thin-layer plate was combined into 6 (A-F) fractions; then choose D to carry out reverse-phase silica gel column chromatography, methanol-water gradient elution (10:90-100:0), and obtain 8 (D1-D8) in total by silica gel thin-layer plate detection ) fractions; select D4 to carry out column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com