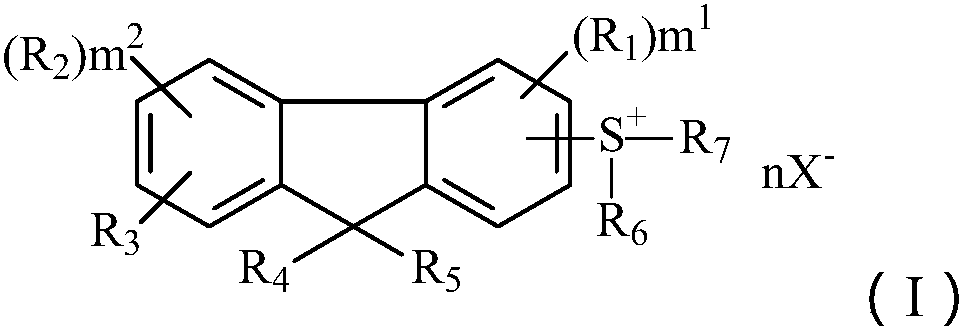
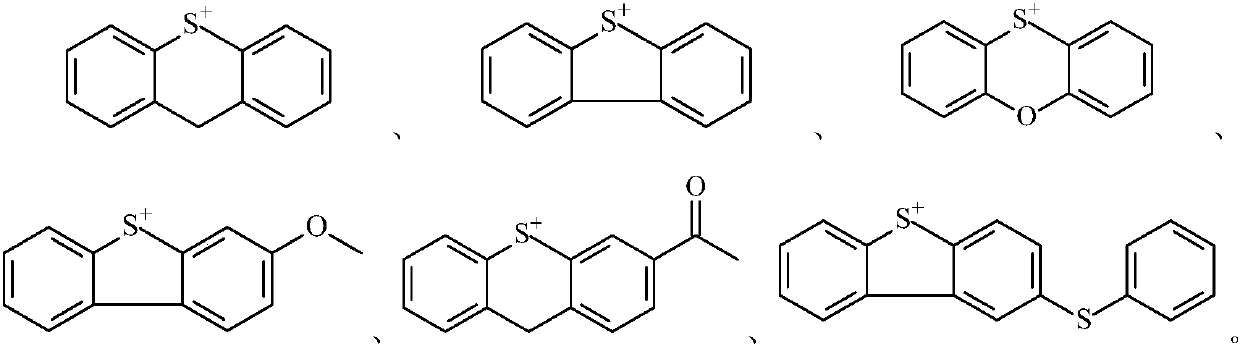
Novel cationic photoinitiator as well as preparation method and application
一种光引发剂、阳离子型的技术,应用在新型阳离子型光引发剂及其制备领域,能够解决不能有效利用长波长光源、易黄变、易迁移出小分子化合物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] (1) Preparation of Intermediate 1a
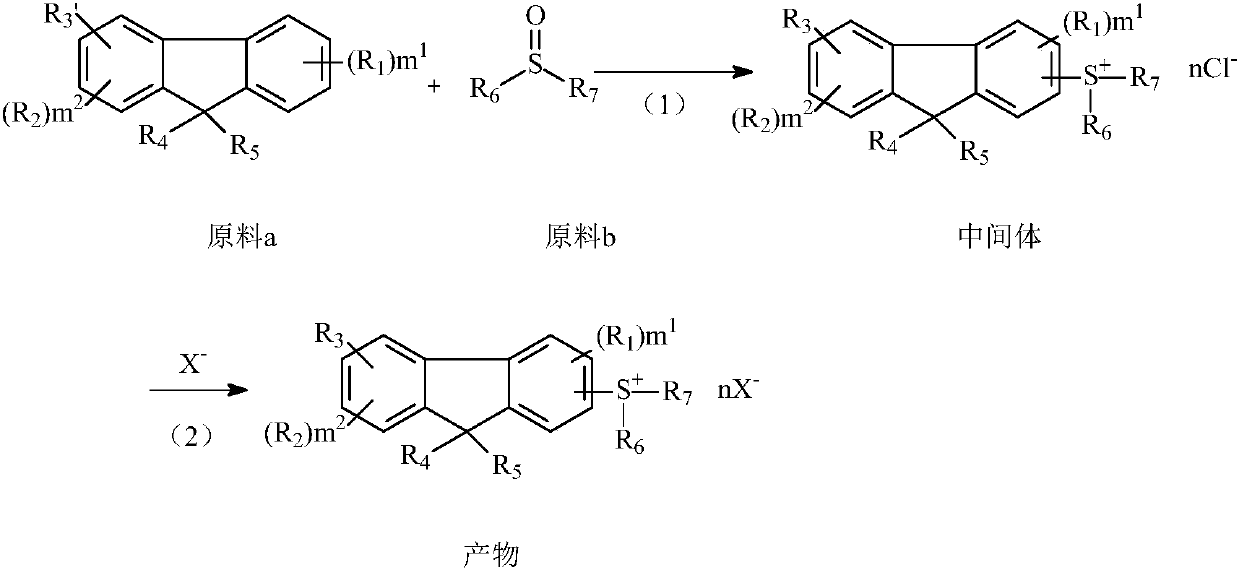
[0055] Add 83g of raw material 1a, 67g of aluminum trichloride, and 200mL of dichloromethane into a 1000mL four-neck flask, and drop the ice-water bath to 0°C. Dissolve 101g of raw material 1b in 200mL of dichloromethane, then put it into a dropping funnel, control the temperature below 10°C, add the mixed solution of raw material 1b and dichloromethane dropwise into the four-neck flask, dropwise after about 2h, add dropwise Continue to stir for 24 hours, track the liquid phase until the concentration of the raw materials does not change, then slowly pour the material into 800 g of deionized water, stir, and the solid precipitates, and suction filters to obtain a light yellow solid, which is dried in an oven at 80 ° C for 2 hours to obtain 152 g of intermediate 1a , yield 79%, purity 98%.
[0056] (2) Preparation of compound 1
[0057] Dissolve 152g of potassium hexafluorophosphate in 150mL of acetone, then add 115g of ...
Embodiment 2
[0062]
[0063] (1) Preparation of Intermediate 2a
[0064] Add 40g of raw material 2a, 14g of aluminum trichloride, and 50mL of dichloromethane into a 500mL four-neck flask, and drop the ice-water bath to 0°C. Dissolve 31g of raw material 2b in 50mL of dichloromethane, then put it into a dropping funnel, control the temperature below 10°C, add the mixed solution of raw material 2b and dichloromethane dropwise into the four-neck flask, dropwise for about 2 hours, add dropwise Continue to stir for 24 hours, track the liquid phase until the concentration of the raw materials does not change, then slowly pour the material into 200 g of deionized water, stir, the solid precipitates, and suction filters to obtain a light yellow solid, which is dried in an oven at 80 ° C for 2 hours to obtain 46 g of intermediate 2a , yield 64%, purity 98%.
[0065] (2) Preparation of Compound 2
[0066] Dissolve 45g of tetrakis(pentafluorophenyl)sodium borate in 100mL of acetone, then add 43g ...
Embodiment 3
[0070]
[0071] (1) Preparation of Intermediate 3a
[0072] Add 22g of raw material 3a, 28g of aluminum trichloride, and 50mL of dichloromethane into a 500mL four-neck flask, and drop the ice-water bath to 0°C. Dissolve 46g of raw material 3b in 100mL of dichloromethane, then put it into a dropping funnel, control the temperature below 10°C, add the mixed solution of raw material 3b and dichloromethane dropwise into the four-neck flask, dropwise for about 2 hours, and dropwise Continue to stir for 24 hours, track the liquid phase until the raw materials no longer change, then slowly pour the materials into 200 g of deionized water, stir, and the solid precipitates, and suction filters to obtain a light yellow solid, which is dried in an oven at 80°C for 2 hours to obtain 56 g of intermediate 3a. Yield 65%, purity 98%.
[0073] (2) Preparation of Compound 3
[0074]Dissolve 60g of sodium perfluorobutane sulfonate in 100mL of acetone, then add 55g of intermediate 3a prepare...
PUM
| Property | Measurement | Unit |
|---|---|---|
| UV absorption wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| Rockwell hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com