Entecavir oral instant film agent and preparation method thereof
An oral instant film agent, entecavir technology, applied in the field of medicine, to achieve the effect of easy film release, stable chemical properties, and good toughness and strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A kind of entecavir oral cavity instant film of the present invention, its each component and its content are as follows:
[0043]
[0044] Wherein, polyvinyl alcohol accounts for 75% of graft copolymer gross weight in polyvinyl alcohol / polyethylene glycol graft copolymer, polyethylene glycol accounts for 25% of graft copolymer gross weight, polyvinyl alcohol / polyethylene glycol The molecular weight of the diol graft copolymer is 45000 Da.
[0045] The preparation method of the above-mentioned entecavir oral instant film is as follows:
[0046] Take the polyvinyl alcohol / polyethylene glycol graft copolymer of the above quality and add it into 10 mL of pure water under stirring, stir and dissolve to obtain a polymer gel. Then add entecavir, glycerin and sodium alginate therein, and stir evenly to obtain a drug-containing solution. The prepared drug-containing solution is left to stand or ultrasonically removed to remove air bubbles, and then evenly coated on a 2cm×1...
Embodiment 2
[0048] A kind of entecavir oral cavity instant film of the present invention, its each component and its content are as follows:
[0049]
[0050] Wherein, polyvinyl alcohol accounts for 75% of graft copolymer gross weight in polyvinyl alcohol / polyethylene glycol graft copolymer, polyethylene glycol accounts for 25% of graft copolymer gross weight, polyvinyl alcohol / polyethylene glycol The molecular weight of the diol graft copolymer is 45000 Da.
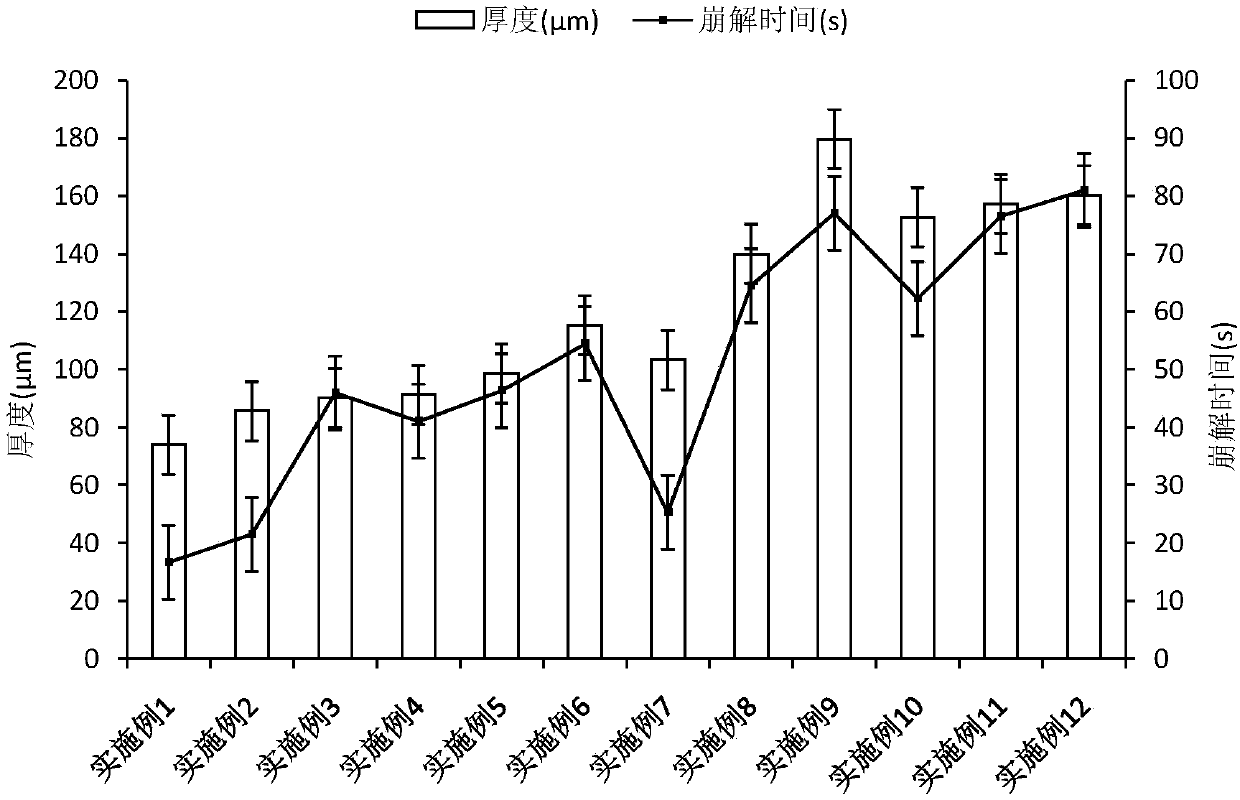
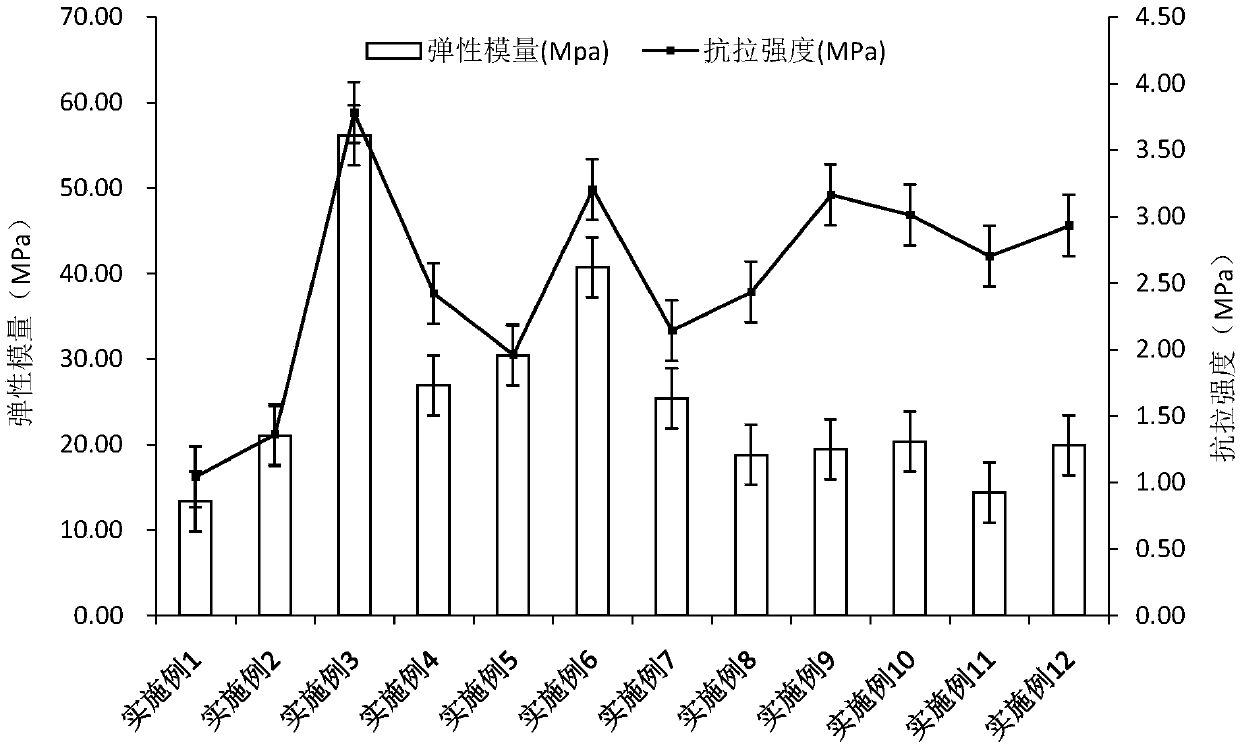
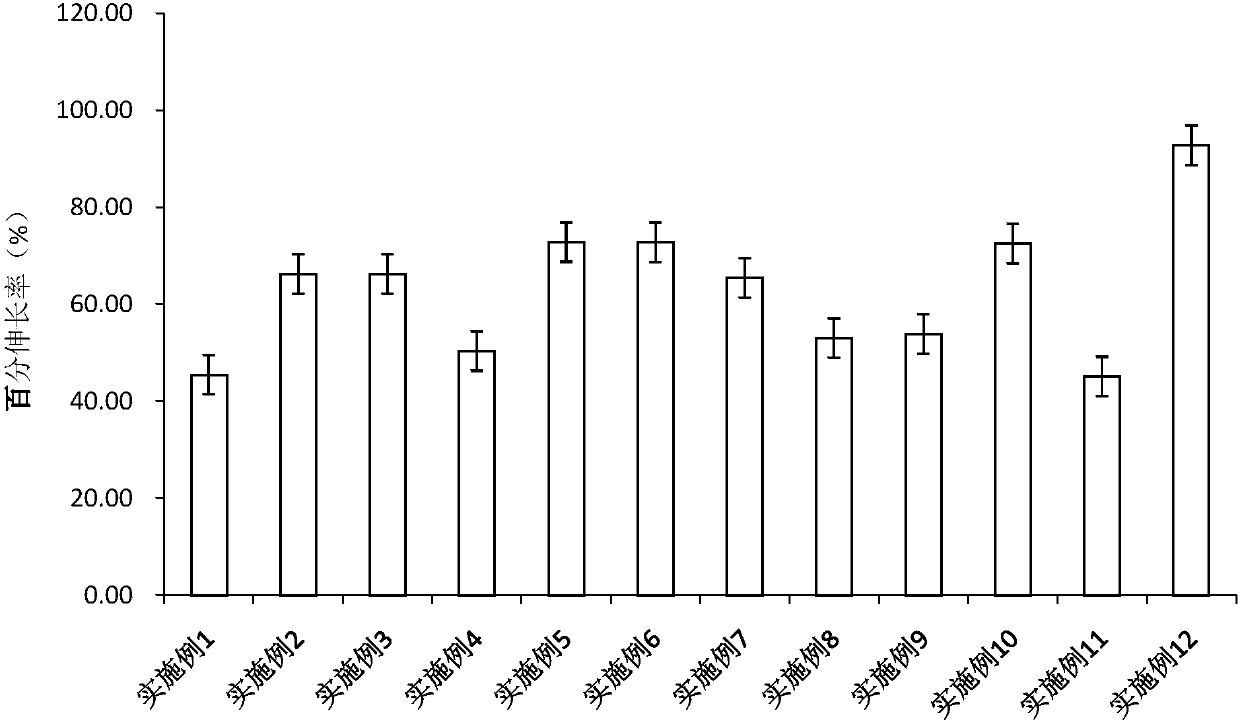
[0051] Entecavir oral cavity instant film was prepared with reference to the method of Example 1. The prepared film agent is transparent, moderate in film thickness, convenient for demoulding, and partially wrinkled.
Embodiment 3
[0053] A kind of entecavir oral cavity instant film of the present invention, its each component and its content are as follows:
[0054]
[0055] Wherein, polyvinyl alcohol accounts for 75% of graft copolymer gross weight in polyvinyl alcohol / polyethylene glycol graft copolymer, polyethylene glycol accounts for 25% of graft copolymer gross weight, polyvinyl alcohol / polyethylene glycol The molecular weight of the diol graft copolymer is 45000 Da.
[0056] Entecavir oral cavity instant film was prepared with reference to the method of Example 1. The prepared film agent is transparent, moderate in film thickness, convenient for demoulding, and partially wrinkled.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com