Chitosan-based bleeding stopping paste as bone wax substitute, and preparation method thereof
A technology of chitosan and substitutes, applied in the field of medicine, to achieve the effect of reducing postoperative infection, good antibacterial performance, and avoiding chronic inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
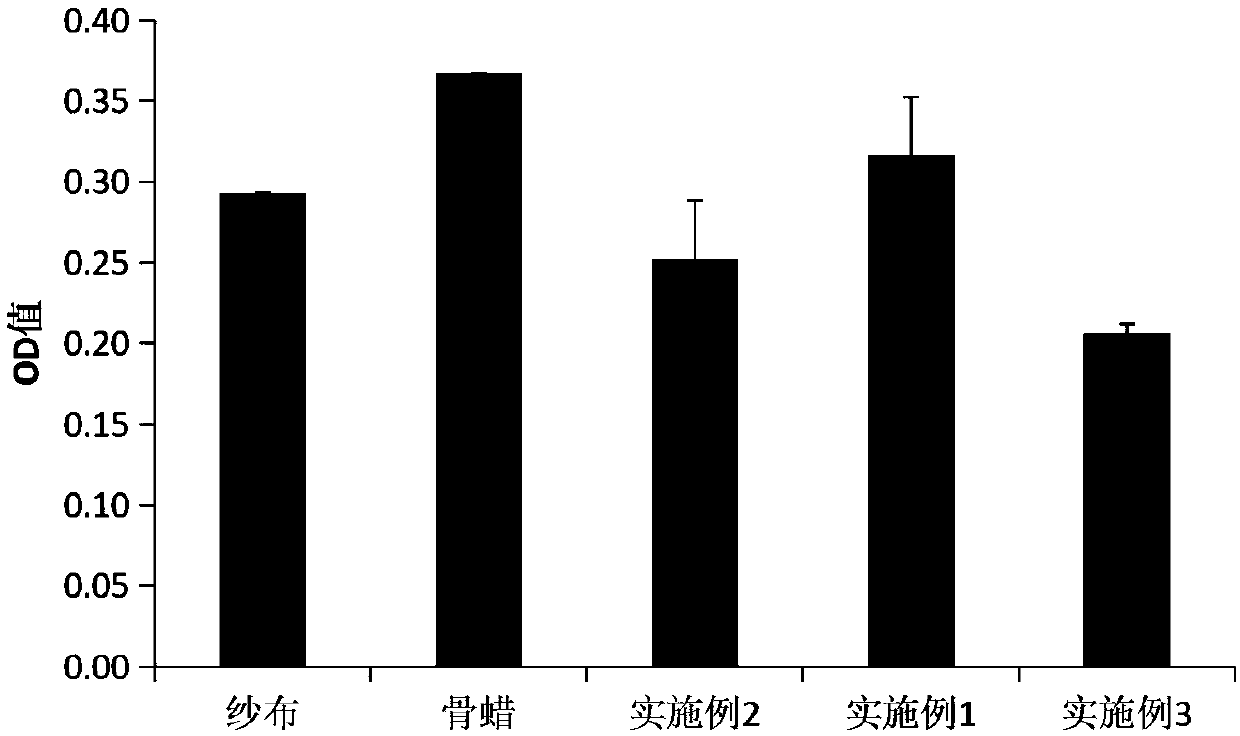
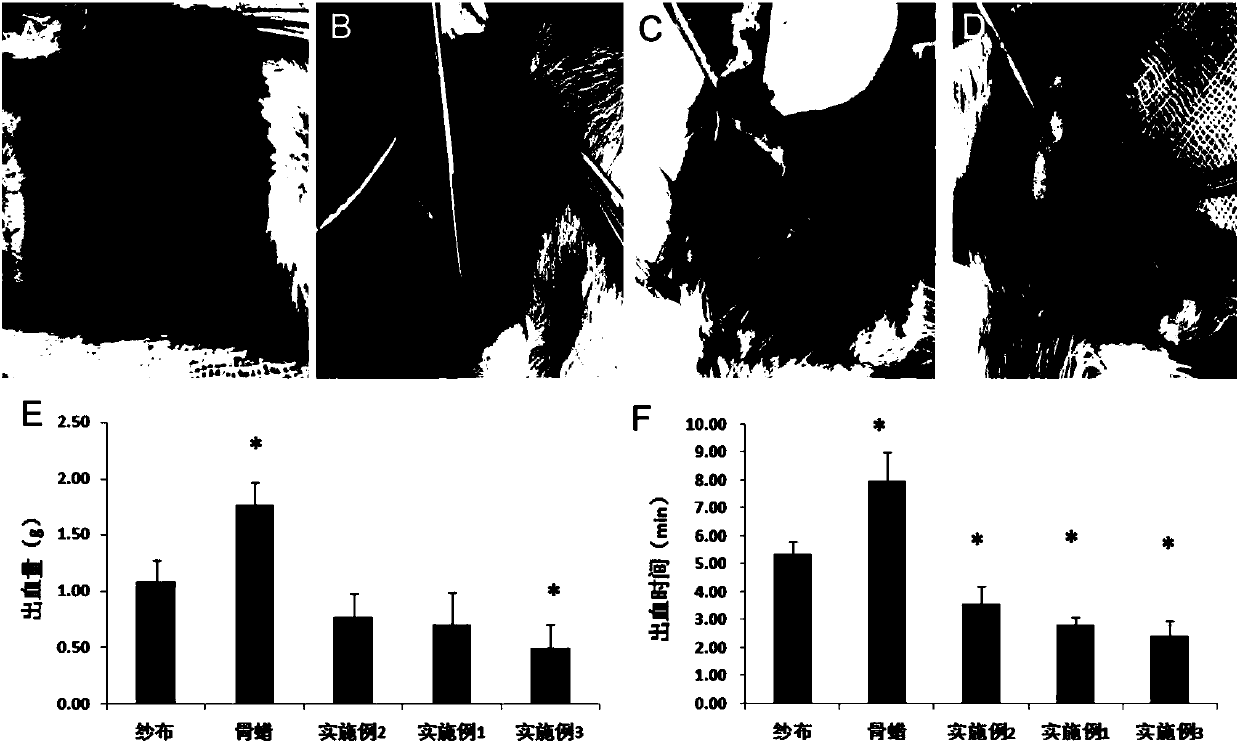
Embodiment 1
[0020] The preparation method of chitosan-based hemostatic paste as a bone wax substitute includes the following steps: dissolving carboxymethyl chitosan with a viscosity average molecular weight of 100,000 and a degree of substitution of 50% in distilled water, and then adding a viscosity average molecular weight of 10 Wan, chitosan and calcium alginate with a degree of deacetylation of 90%, stir well and seal, and sterilize with ethylene oxide; the mass ratio of carboxymethyl chitosan, distilled water, chitosan and calcium alginate is 2:100:15:45.
Embodiment 2
[0022] The preparation method of chitosan-based hemostatic paste as a bone wax substitute includes the following steps: dissolving carboxymethyl chitosan with a viscosity average molecular weight of 150,000 and a degree of substitution of 150% in distilled water, and then adding a viscosity average molecular weight of 30 Wan, chitosan and calcium alginate with a degree of deacetylation of 90%, stir well and seal, and sterilize with ethylene oxide; the mass ratio of carboxymethyl chitosan, distilled water, chitosan and calcium alginate is 6:100:45:15.
Embodiment 3
[0024] The preparation method of chitosan-based hemostatic paste as a substitute for bone wax includes the following steps: dissolving carboxymethyl chitosan with a viscosity average molecular weight of 120,000 and a degree of substitution of 65% in distilled water, and then adding a viscosity average molecular weight of 15 10,000. Chitosan and calcium alginate with a degree of deacetylation of 90%, stir well and seal, and sterilize with ethylene oxide; the mass ratio of carboxymethyl chitosan, distilled water, chitosan and calcium alginate is 4:100:30:30.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com