Preparation method of chemical/ionic double-crosslinking interpenetration network hydrogel
An interpenetrating network and ionic cross-linking technology, which is applied in the field of preparation of high-strength chemical/ionic double cross-linked interpenetrating network hydrogels, can solve problems such as hydrogel instability, and achieve improved mechanical properties and biocompatibility properties, mild reaction conditions, and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
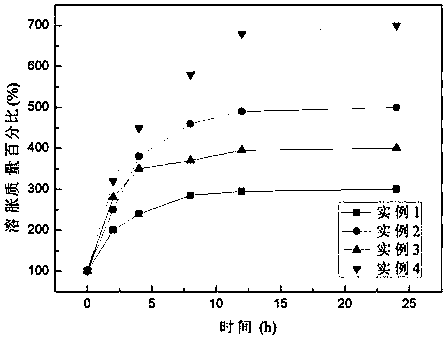
Examples
Embodiment 1
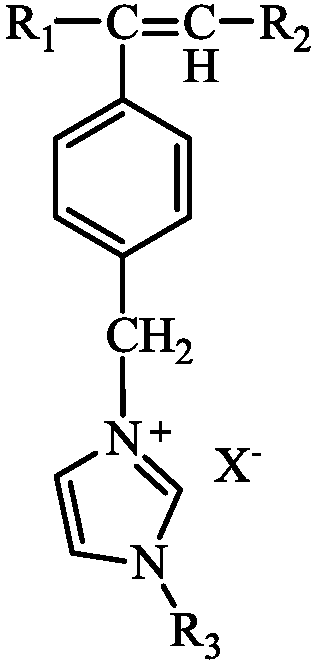
[0031] The specific preparation method is as follows: (1) Weigh 0.01mol 4-chloromethylstyrene (VC) and 0.01mol acrylamide into a three-necked flask containing 5mL N,N-dimethylformamide, magnetically stir and mix evenly, Add 0.015g of azobisisobutyronitrile, under the protection of nitrogen, heat, stir and reflux in an oil bath at 65°C for 12 hours. After the reaction, transfer the viscous liquid to a beaker and dry it in vacuum at 60°C for 8 hours. Polymer A was obtained after repeated washing and drying of ether.
[0032] (2) Weigh 0.01mol 1-vinylimidazole (VM), 0.01mol acrylamide and 0.02mol 1-(4-vinylbenzyl)-3-methylimidazole In a three-necked flask of methylformamide, magnetically stir and mix evenly, add 0.015g of azobisisobutyronitrile, under the protection of nitrogen, heat and stir in an oil bath at 65°C for reflux reaction for 12h, after the reaction, transfer the viscous liquid to In a beaker, vacuum-dry at 60°C for 8 hours, and the dried product is repeatedly washe...
Embodiment 2
[0035] The specific preparation method is: (1) Weigh 0.01mol 4-chloromethylstyrene (VC) and 0.02mol N,N-dimethylacrylamide into a three-necked flask containing 6mL N,N-dimethylformamide , stir and mix evenly with magnetic force, add 0.015g of azobisisobutyronitrile, under the protection of nitrogen, heat, stir and reflux in an oil bath at 65°C for 12 hours, transfer the viscous liquid to a beaker after the reaction, and vacuum at 60°C After drying for 8 hours, the dried product was repeatedly washed and dried with petroleum ether to obtain polymer A.
[0036](2) Weigh 0.01mol 1-vinylimidazole (VM), 0.02mol N,N-dimethylacrylamide and 0.04mol 1-(4-vinylbenzyl)-3-methyl imidazole chloride into the In a three-neck flask of 12mL N,N-dimethylformamide, magnetically stir and mix evenly, add 0.02g of azobisisobutyronitrile, under the protection of nitrogen, heat, stir and reflux in an oil bath at 65°C for 12h, after the reaction The viscous liquid was transferred to a beaker and drie...
Embodiment 3
[0039] The specific preparation method is as follows: (1) Weigh 0.01mol 4-chloromethylstyrene (VC) and 0.05mol acrylamide into a three-necked flask containing 10mL N,N-dimethylformamide, magnetically stir and mix evenly, Add 0.02g of azobisisobutyronitrile, under the protection of nitrogen, heat, stir and reflux in an oil bath at 65°C for 16 hours. After the reaction, transfer the viscous liquid to a beaker and dry it in vacuum at 60°C for 8 hours. Polymer A was obtained after repeated washing and drying of ether.
[0040] (2) Weigh 0.01mol 1-vinylimidazole (VM), 0.02mol acrylamide and 0.03mol 1-(4-vinylbenzyl)-3-methylimidazole In a three-necked flask of methylformamide, magnetically stir and mix evenly, add 0.02g of azobisisobutyronitrile, under the protection of nitrogen, heat and stir in an oil bath at 65°C for reflux reaction for 16h, and transfer the viscous liquid to In a beaker, vacuum-dry at 60°C for 8 hours, and the dried product is repeatedly washed and dried with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com