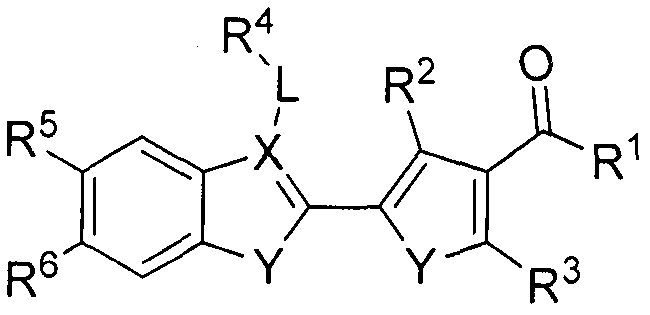
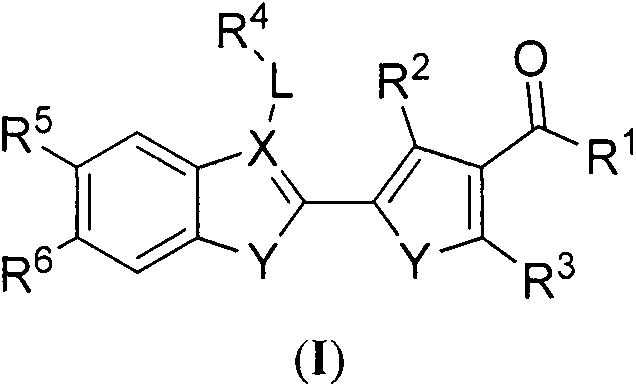
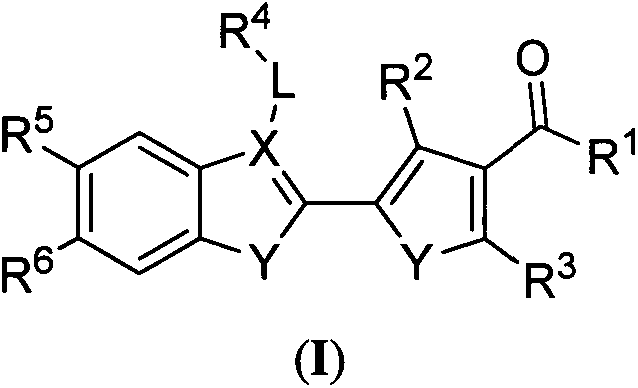
Preparation method and purpose of pyrrolone BRD4 protein inhibitor
A technology of pyrrole and ethyl ketone, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve many problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] 3,5-Dimethyl-4-acetyl-1H-pyrrole-2-carboxylic acid ethyl ester (I-a)
[0183] Add 9.80 g (75.04 mmol) of ethyl acetoacetate and 20 mL of glacial acetic acid into a 250 mL eggplant-shaped bottle in sequence, and place in an ice bath
[0184] N-(2-(3,5-Dimethyl-4-acetyl-1H-pyrrol-2-yl)-1H-indol-3-yl)benzamide (II-7)
[0185] N-(2-(3,5-Dimethyl-4-acetyl-1H-pyrrol-2-yl)-IH-indol-3-yl)cyclohexanecarboxamide (II-8)
[0186] N-(2-(3,5-Dimethyl-4-acetyl-1H-pyrrol-2-yl)-1H-indol-3-yl)cyclopropanecarboxamide (II-9)
[0187] N-(2-(3,5-Dimethyl-4-acetyl-1H-pyrrol-2-yl)-1H-indol-3-yl)-2-methoxybenzamide (II-10)
[0188] 1-(1,2,4-Trimethyl-5-(1H-indol-2-yl)-1H-pyrrol-3-yl)ethanone (II-11)
[0189] 1-(2,4-Dimethyl-5-(5-morpholino-1H-indol-2-yl)-1H-pyrrol-3-yl)ethanone (II-12)
[0190] 1-(2,4-Dimethyl-5-(3-(pyridin-2-ylamino)-1H-indol-2-yl)-1H-pyrrol-3-yl)ethanone (II-13)
[0191] 1-(2,4-Dimethyl-5-(3-(phenylamino)-1H-indol-2-yl)-1H-pyrrol-3-yl)ethanone (II-14)
[0192] 1-(2,4-Di...
Embodiment 5
[0207] 1-(1,2,4-trimethyl-5-(1-methyl-1H-phenyl[d]imidazol-2-yl)-1H-pyrrol-3-yl)ethanone (I-33 )
[0208]Put 160mg (0.63mmol) of compound I-1 in a 50mL one-mouth bottle with anhydrous DMF solvent, stir in an ice bath and add 50mL NaHH (1.26mmol), add CH3I 0.051mL (0.82mmol) after 20mins, react at room temperature for 5h, TLC detects that the reaction is complete . The reaction solution was poured into 100 mL of water, extracted with ethyl acetate (50 mL×3), the organic phases were combined, washed with 100 mL of saturated brine, dried over anhydrous sodium sulfate, and left to stand. After filtration, the solvent was distilled off under reduced pressure and separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:9) to obtain 148 mg of solid with a yield of 83.44%. ESI-MS m / z: 304.3[M+Na] + . 1 H-NMR (300MHz, DMSO-d 6 )δ: 7.70-7.61 (m, 2H, ArH), 7.21-7.15 (m, 2H, ArH), 3.94 (s, 3H, -CH 3 ), 3.54(s, 3H, -CH 3 ), 2.48(s, 3H, O=C-CH 3 ), 2.43 (s, 3H,...
Embodiment 6
[0210] 2-(4-Acetyl-3,5-dimethyl-1H-pyrrol-2-yl)-1H-benzo[d]imidazole-6-carboxylic acid (I-d)
[0211] Add 165mg (1.00mmol) of compound (I-c), 152mg (1.00mmol) of 3,4-diaminobenzoic acid, 19mg (0.10mmol) of sodium metabisulfite, and 10mL of ethanol into a 50mL two-necked flask in turn, under nitrogen protection, and heat at 90°C After 5 h, TLC detected that the reaction was complete. After cooling, the reaction solution was poured into 100 mL of water, extracted with ethyl acetate (50 mL×3), the organic phases were combined, washed with 100 mL of saturated brine, dried over anhydrous sodium sulfate, and left to stand. After filtration, the solvent was distilled off under reduced pressure and separated by silica gel column chromatography (ethyl acetate:petroleum ether=1:1) to obtain 266 mg of an orange-yellow solid with a yield of 89.47%. ESI-MS m / z: 320.2[M+Na] + . 1 H-NMR (300MHz, DMSO-d 6 )δ: 12.38 (s, 1H, -COOH), 12.21 (s, 1H, -NH), 10.92 (s, 1H, -NH), 8.41 (s, 1H, ArH),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com