Production method of high-purity 2-chloro-5-trifluoromethylpyridine
A technology for trifluoromethylpyridine and crude trifluoromethylpyridine, which is applied in the production field of 2-chloro-5-trifluoromethylpyridine, can solve the problems of many by-products, difficult purification, similar properties and the like, and achieves production The effect of good capacity, high product purity and stable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
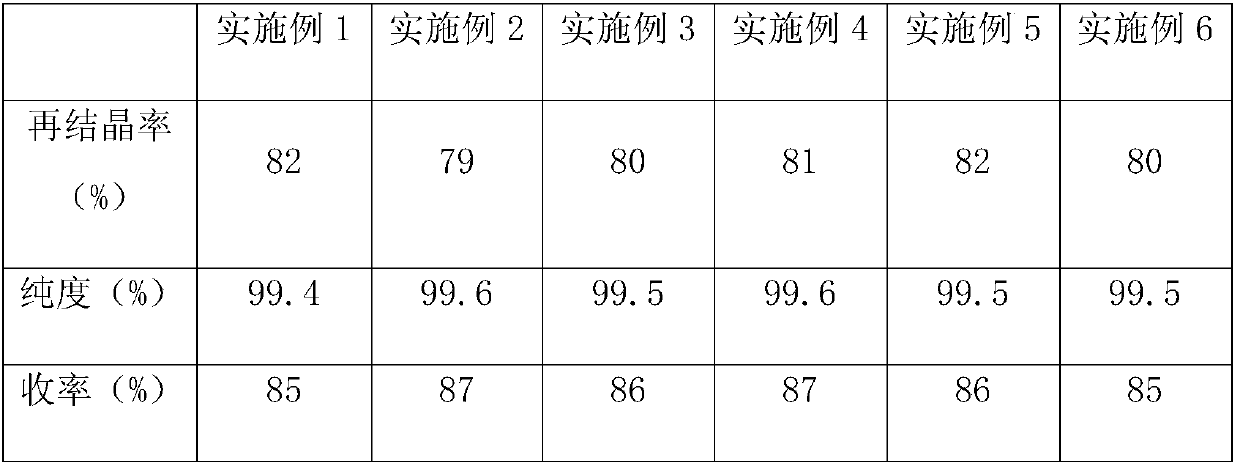
Examples
Embodiment 1
[0023] (1) Under the action of silica-aluminum trioxide-chromium trioxide catalyst, acrolein and propionaldehyde are used as raw materials, after gasification, react with ammonia gas at 440°C for 1 hour, and the yield is not less than 65% 3-picoline.
[0024] (2) After the co-gasification of 3-picoline and carbon tetrachloride, react under chlorine atmosphere to obtain 2-chloro-5-trichloromethylpyridine, under the catalyst is mercuric oxide, carry out fluorine substitution with hydrogen fluoride, The yield of crude 2-chloro-5-trifluoromethylpyridine was not less than 85%.
[0025] (3) After heating and melting the crude 2-chloro-5-trifluoromethylpyridine at a rate of 0.5 °C / min to 29 °C, it was placed in a low-temperature constant temperature device for refrigeration and circulation, and cooled at a rate of 0.07 °C / min Crystallize at 14°C, separate to obtain recrystallization, sweat the recrystallization at 27°C, repeat the process of melting, recrystallization and sweating t...
Embodiment 2
[0027] (1) Under the action of silica-aluminum trioxide-chromium trioxide catalyst, acrolein and propionaldehyde are used as raw materials, after gasification, react with ammonia gas at 445°C for 3 hours, and the yield is not less than 65% 3-picoline.
[0028] (2) After the co-gasification of 3-picoline and carbon tetrachloride, react under chlorine atmosphere to obtain 2-chloro-5-trichloromethylpyridine, under the catalyst is mercuric oxide, carry out fluorine substitution with hydrogen fluoride, The yield of crude 2-chloro-5-trifluoromethylpyridine was not less than 85%.
[0029] (3) After heating and melting the crude 2-chloro-5-trifluoromethylpyridine at a rate of 0.7 °C / min to 33 °C, it was placed in a low-temperature constant temperature device for refrigeration and circulation, and cooled at a rate of 0.07 °C / min Crystallize at 22°C, separate to obtain recrystallization, sweat the recrystallization at 30°C, repeat the process of melting, recrystallization and sweating ...
Embodiment 3
[0031] (1) Under the action of silica-aluminum trioxide-chromium trioxide catalyst, acrolein and propionaldehyde are used as raw materials, after gasification, react with ammonia gas at 442°C for 2 hours, and the yield is not less than 65% 3-picoline.
[0032] (2) After the co-gasification of 3-picoline and carbon tetrachloride, react under chlorine atmosphere to obtain 2-chloro-5-trichloromethylpyridine, under the catalyst is mercuric oxide, carry out fluorine substitution with hydrogen fluoride, The yield of crude 2-chloro-5-trifluoromethylpyridine was not less than 85%.
[0033] (3) After heating and melting the crude 2-chloro-5-trifluoromethylpyridine at a rate of 0.6 °C / min to 30 °C, it was placed in a low-temperature constant temperature device for refrigeration and circulation, and cooled at a rate of 0.07 °C / min Crystallize at 16°C, separate to obtain recrystallization, sweat the recrystallization at 28°C, repeat the process of melting, recrystallization and sweating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com