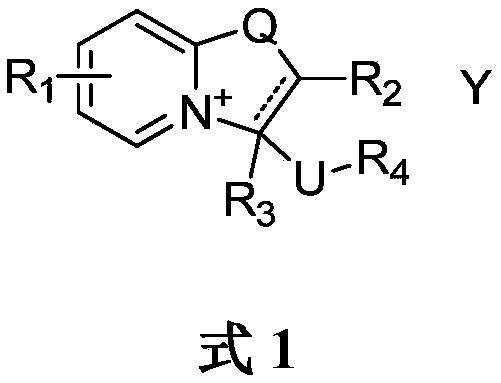
Oxazolopyridine quaternary ammonium salt compound and preparation method and application thereof
A technology of oxazolopyridine and quaternary ammonium salts, which is applied in the transformation application field of oxazolopyridine quaternary ammonium salt compounds and synthetic intermediates, can solve the problem of low yield, achieve convenient post-processing, and good promotion and application value , The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
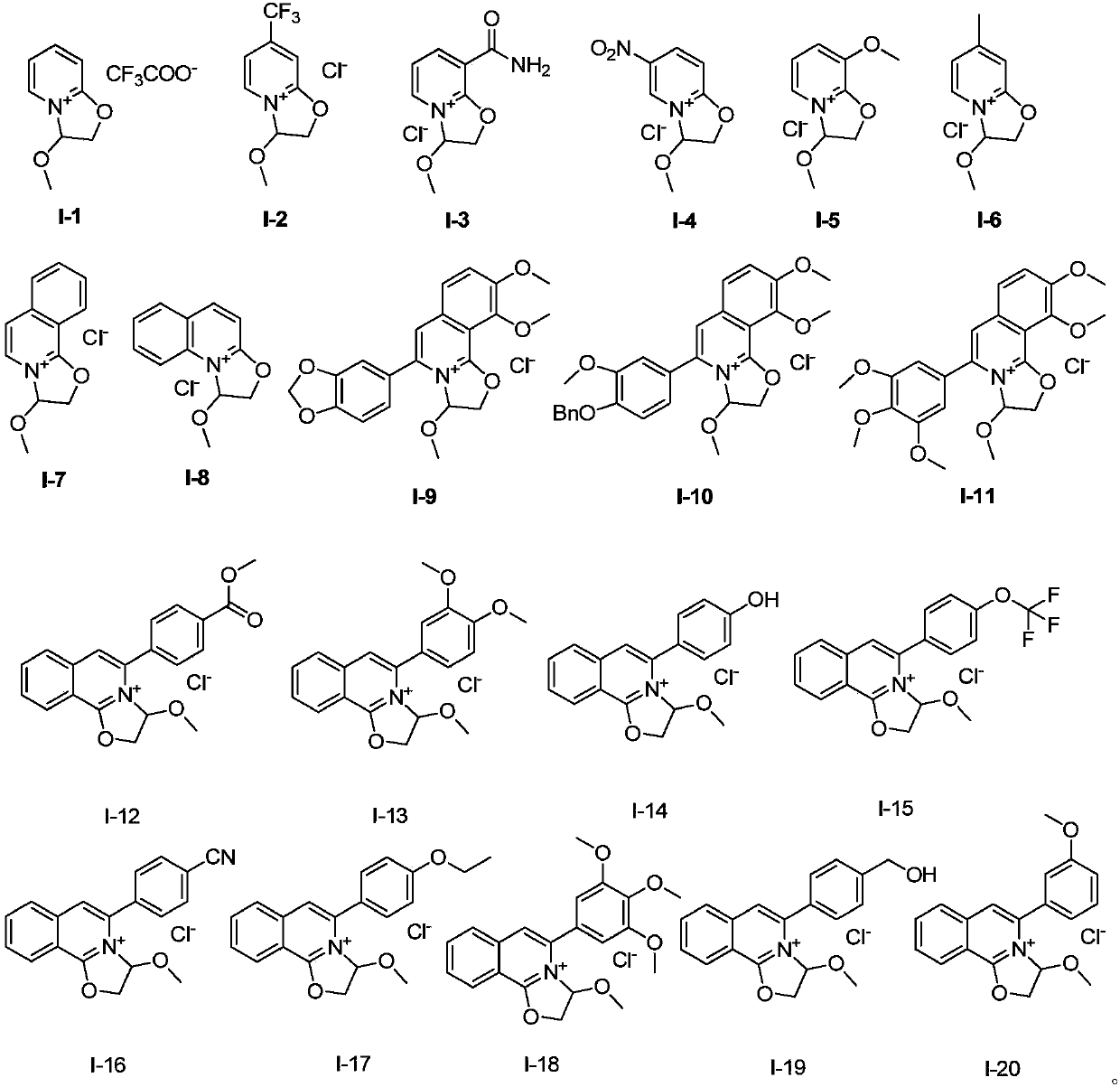
[0048] The preparation of embodiment 1 compound I-1
[0049]
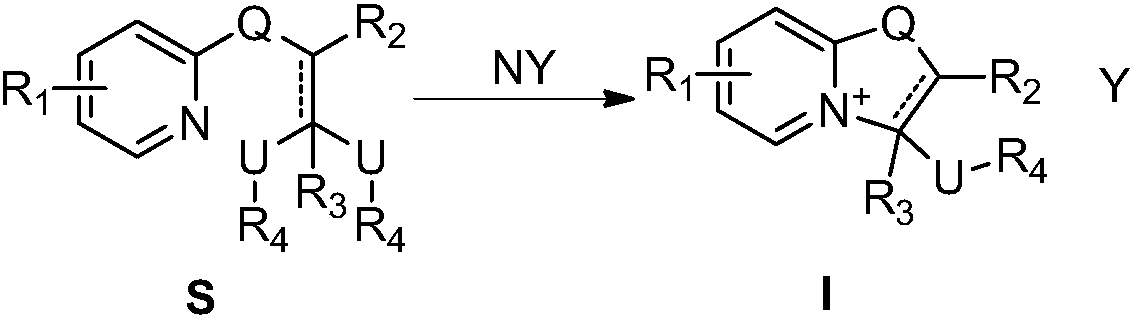
[0050] Dissolve compound S-1 in dry toluene, add excess trifluoroacetic acid to it, heat to 50°C, keep dry, react overnight, spin dry excess acid and solvent, and wash with ether to obtain pure compound I-1 , and the yield was 86%. 1 HNMR (400MHz, chloroform-d) δ7.62 (dd, J = 7.0, 2.0Hz, 1H), 7.52 (ddt, J = 8.7, 6.6, 2.7Hz, 1H), 6.75 (d, J = 9.1Hz, 1H ),6.47(q,J=7.1Hz,1H),6.08–5.97(m,1H),3.78(q,J=11.5,9.8Hz,2H),3.37(s,3H). 13 C NMR (125MHz, CDCl 3 )δ159.91, 149.11, 137.12, 119.27, 110.64, 93.01, 75.74, 58.15. MS (EI): 265.
Embodiment 2
[0051] The preparation of embodiment 2 compound 1-2
[0052]
[0053] Compound S-2 was dissolved in dry anhydrous ether, and excessive concentrated hydrochloric acid was added thereto, and a large amount of yellow solid was precipitated immediately. After stirring for 3 hours, it was filtered and washed with anhydrous ether to obtain pure compound I-2. The yield was 92%. 1 H NMR (400MHz, chloroform-d) δ7.66 (d, J = 7.3Hz, 1H), 6.88 (dd, J = 1.9, 1.0Hz, 1H), 6.43 (dd, J = 7.3, 2.0Hz, 1H) ,6.07(dd,J=4.8,3.6Hz,1H),3.87–3.70(m,2H),3.45(s,3H). 13 C NMR (125MHz, CDCl 3 )δ160.93, 141.24, 140.97, 133.41, 122.63, 120.45, 117.99, 100.85, 84.91, 57.37, 43.98. MS(EI) 255.
Embodiment 3
[0054] The preparation of embodiment 3 compound 1-3
[0055]
[0056] The preparation method was the same as in Example 2, except that compound S-2 was replaced by compound S-3 to obtain compound I-3 with a yield of 99%. 1 HNMR (400MHz, chloroform-d) δ9.28(s, 1H), 8.60(dd, J=7.2, 2.2Hz, 1H), 7.77(dd, J=6.8, 1.9Hz, 1H), 6.56(t, J =6.9Hz,1H),6.22–6.09(m,1H),5.97(s,1H),3.93–3.69(m,2H),3.46(s,3H). 13 C NMR (125MHz, CDCl 3 )δ164.76, 161.45, 144.37, 135.61, 120.97, 106.16, 85.37, 57.49, 44.09. MS (ESI) 195.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com