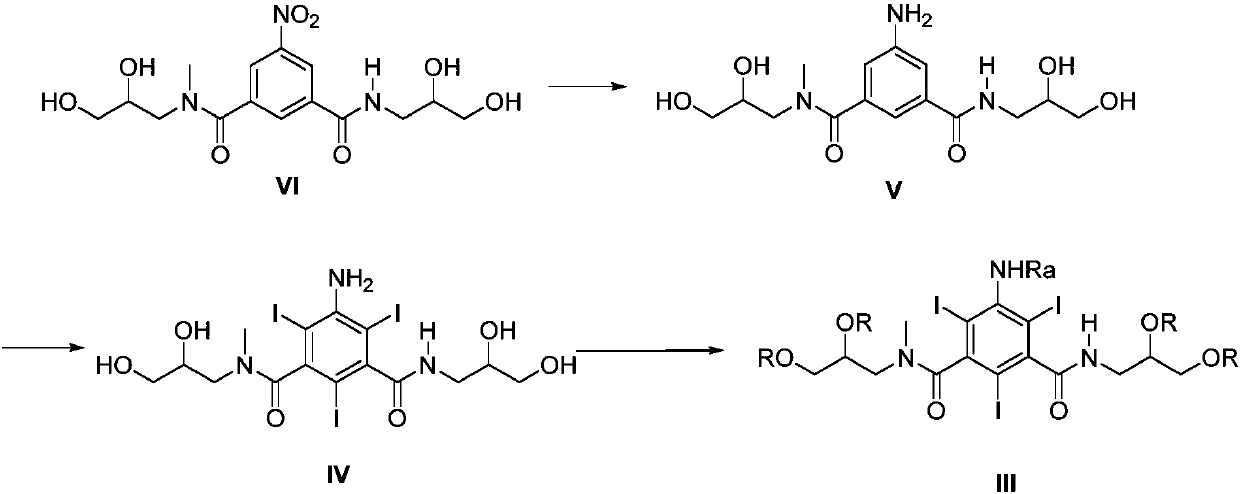
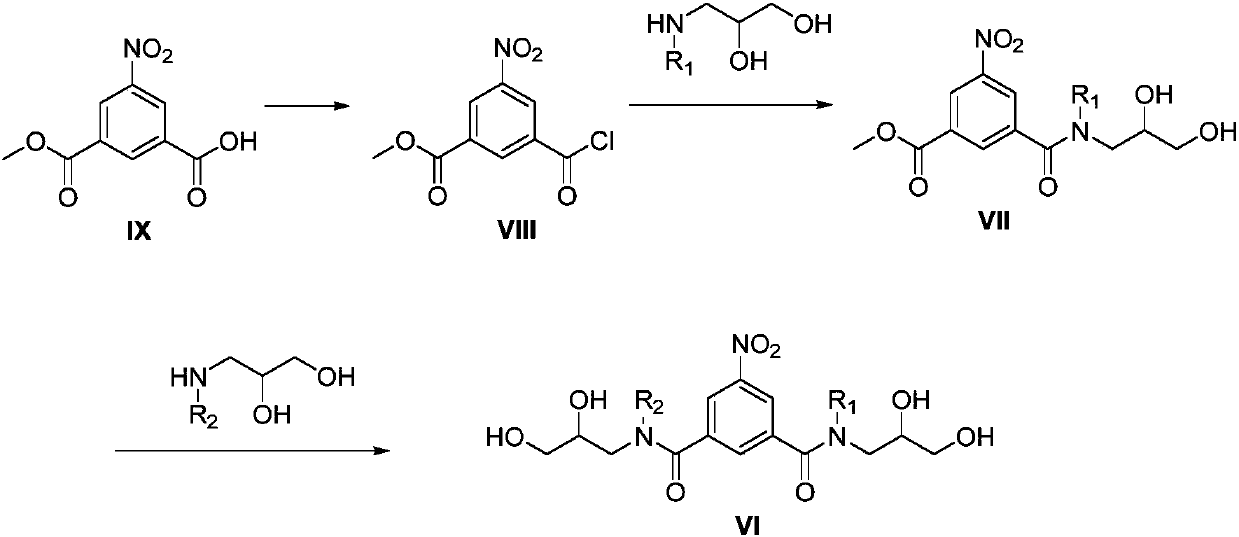
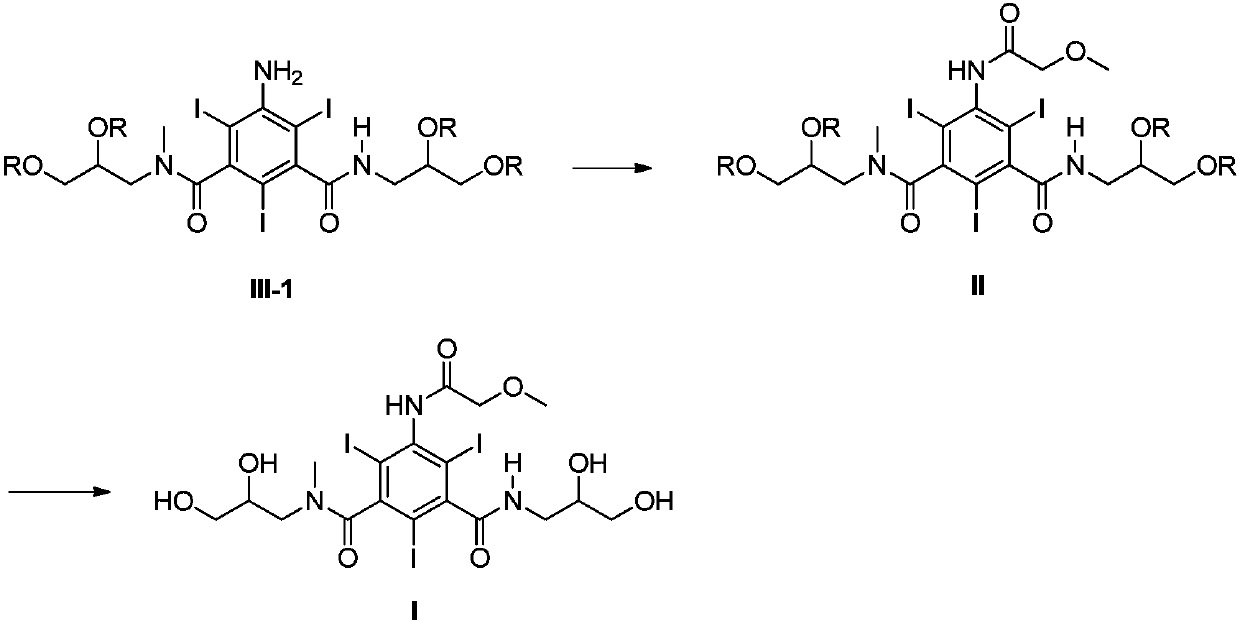
Preparation methods of iopromide and intermediate of iopromide
A technology of iopromide and iodination, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of many by-products in the iodination reaction step, unfavorable industrial production, and difficult separation and purification. , to achieve the effects of easy separation and purification, reduction of by-product formation, and avoidance of diacylated by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the preparation of 2-methoxyacetyl chloride
[0056]
[0057] 2-Methoxyacetic acid (656.7mmol), dichloromethane (197mL) and DMF (21.9mmol) were added into a 500mL three-neck flask, the reaction solution was cooled to 10°C, and thionyl chloride (525.3mmol ), dropwise, react at room temperature for 12 hours, and concentrate the reaction solution at 25°C to obtain 2-methoxyacetyl chloride.
Embodiment 2
[0058] Embodiment 2: Preparation of 3-chloroformyl-5-nitrobenzoic acid methyl ester (compound of formula VIII)
[0059]
[0060] Add the compound of formula IX (66.62mmol) into a 250mL three-necked flask, add dichloromethane (45mL) and DMF (0.075mL), heat in a water bath at 25°C, disperse for 10 minutes, slowly add oxalyl chloride (99.9mmol) dropwise, and react After the liquid was clarified, continue to stir for half an hour, then concentrate, add dichloromethane (15mL×2) and continue to concentrate twice to obtain a white solid compound 3-chloroformyl-5-nitrobenzoic acid methyl ester, add di Chloromethane (37 mL) was dissolved for use.
Embodiment 3
[0061] Embodiment 3: Preparation of 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid methyl ester (compound of formula VII-1)
[0062]
[0063] Add aminoglycerol (159.9mmol) and absolute ethanol (22.5mL) into a three-neck flask, stir at room temperature to dissolve, then cool the reaction solution to -30°C-20°C, and slowly add the dichloromethane solution of the compound of formula VIII dropwise During the dropping process, the temperature of the feed liquid is controlled not to be higher than -13°C, and the stirring is continued for 0.5 hours after the dropping. After the reaction, add 1 mol / L dilute hydrochloric acid dropwise to adjust the pH to 1-2, stir for half an hour, warm up to room temperature, separate the liquids, wash the organic phase with 60 mL of water, combine the two water phases, add a mixture of dichloromethane / ethanol Extract twice with solvent (dichloromethane / ethanol=2:1) (60mL×2), combine the organic phases, add anhydrous sodium sulfate to dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com