Pd/UiO-66 catalyst having morphology-controllable Pd metal nanocrystal core and preparation method thereof
A technology of metal nanocrystals and catalysts, applied in the field of nanomaterials, can solve problems such as limiting the application of catalysts, and achieve significant research significance and value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
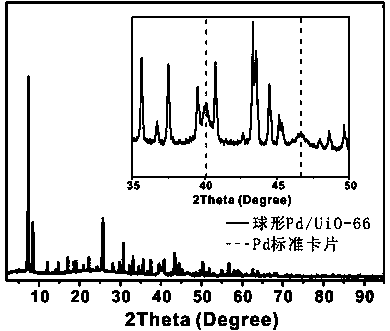
[0034] Example 1 A Pd / UiO-66 catalyst with spherical morphology Pd metal nanocrystal core I
[0035] A Pd / UiO-66 catalyst with a Pd metal nanocrystal core with spherical morphology, the preparation method of the Pd / UiO-66 catalyst is as follows:
[0036] (1) Weigh 37 mg of terephthalic acid (H 2 BDC), mixed with ultrasonic at room temperature and dissolved in 4 mL of N,N-dimethylformamide (DMF) to form solution A; weigh 10 mg of sodium chloropalladate (Na 2 PdCl 4 ), dissolved in 2 mL of N,N-dimethylformamide (DMF) at room temperature by ultrasonic mixing to form solution B; weigh 52 mg of zirconium tetrachloride (ZrCl 4 ), dissolved in 4 mL of N,N-dimethylformamide (DMF) by ultrasonic mixing at room temperature to form solution C;
[0037] (2) Mix solution B and solution A, stir for 5 min, add solution C to the above mixed solution at room temperature, and then add 1.2 mL of acetic acid to the mixed solution.
[0038] (3) The above mixed solution was sealed and stirred in...
Embodiment 2
[0039] Example 2 A Pd / UiO-66 catalyst with spherical morphology Pd metal nanocrystal core II
[0040] The preparation method of the catalyst provided in this example is the same as that in Example 1, except that in this example, in step (1), the amount of zirconium tetrachloride is 52 mg, and the amount of terephthalic acid is 37 mg. The amount of sodium chloropalladate was 10 mg; the temperature of the oil bath in step (3) was 115° C., and the stirring time was 22 hours.
Embodiment 3
[0041] Example 3 A Pd / UiO-66 Catalyst I with Tetrahedral Morphology and Pd Metal Nanocrystal Core
[0042] A Pd / UiO-66 catalyst with a Pd metal nanocrystal core with tetrahedral morphology, the preparation method of the Pd / UiO-66 catalyst is as follows:
[0043] (1) Weigh 37 mg of terephthalic acid (H 2 BDC), ultrasonically mixed and dissolved in 4 mL of N,N-dimethylformamide (DMF) at room temperature to form solution A; weigh 10 mg of palladium acetylacetonate (Pd(acac) 2 ), dissolved in 2 mL of N,N-dimethylformamide (DMF) at room temperature by ultrasonic mixing to form solution B; weigh 52 mg of zirconium tetrachloride (ZrCl 4 ), dissolved in 4 mL of N,N-dimethylformamide (DMF) by ultrasonic mixing at room temperature to form solution C;
[0044](2) Mix solution B and solution A, stir for 5 min, add solution C to the above mixed solution at room temperature, and then add 1.2 mL of acetic acid to the mixed solution.
[0045] (3) The above mixed solution was sealed and sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Side length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com