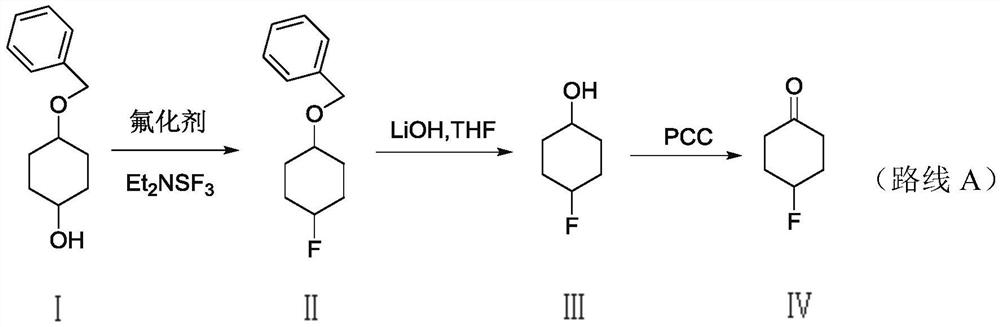
A kind of method for synthesizing 4-fluorocyclohexanone
A technology of fluorocyclohexanone and cyclohexanedione monoethylene glycol ketal, which is applied in the field of synthesis of fluorine-containing compounds, can solve problems such as inability to meet the needs of industrial production, difficulty in large-scale industrial production, and cumbersome synthetic routes. Achieve the effects of saving production time and cost, simplifying the post-processing process, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
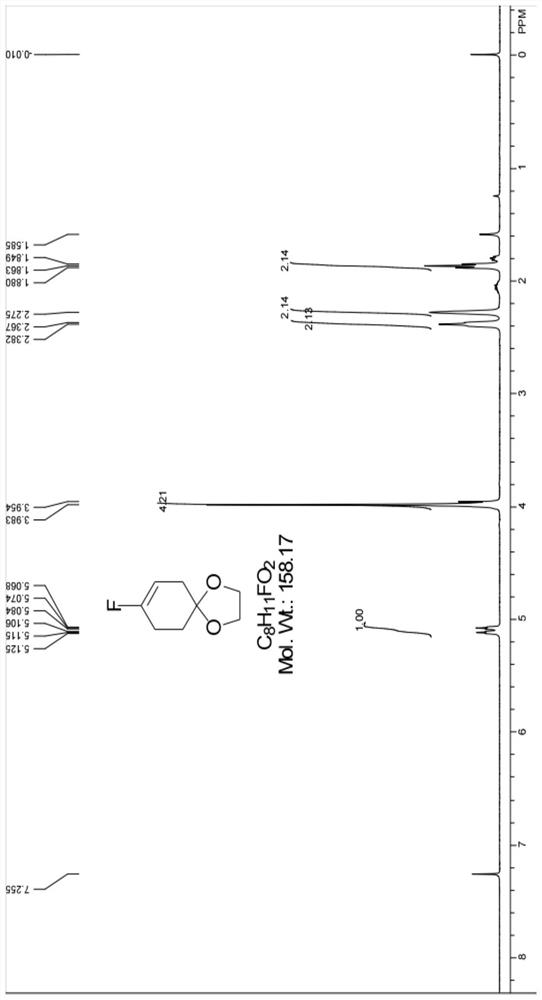
[0045] (1) Synthesis of 8-fluoro-1,4-dioxaspiro[4.5]dec-7-ene:
[0046] Add 312g of dichloromethane, pyridine (237.3g, 3.0mol, 3.0eq), and 1,4-cyclohexanedione monoethylene glycol ketal (156.2g, 1.0mol, 1.0eq) into a 1000ml three-necked reaction flask in sequence .
[0047] Start the stirring, lower the temperature to 10±5°C, add DAST (193.4g, 1.2mol, 1.2eq) dropwise, control the internal temperature at 10±5°C, after the dropwise addition, raise the temperature by 5°C per hour to 25°C, and The reaction was carried out at 25°C for 12 hours. GC central control reaction process: GC central control monitors that the peak integral area of raw materials on the GC spectrum / (integrated area of raw material peak + product peak integral area) < 0.5%, that is, the GC central control monitors that the remaining amount of reaction raw materials < 0.5%, to end the reaction, the raw material here refers to 1,4-cyclohexanedione monoethylene glycol ketal, and the product here refers to...
Embodiment 2
[0058] (1) Synthesis of 8-fluoro-1,4-dioxaspiro[4.5]dec-7-ene
[0059] Add 312g of dichloromethane, triethylamine (303.6g, 3.0mol, 3.0eq), 1,4-cyclohexanedione monoethylene glycol ketal (156.2g, 1.0mol, 1.0eq) into 1000ml three-port reaction in sequence in the bottle.
[0060] Start the stirring, lower the temperature to 10±5°C, add DAST (193.4g, 1.2mol, 1.2eq) dropwise, control the internal temperature at 10±5°C, after the dropwise addition, raise the temperature by 5°C per hour to 25°C, and The reaction was carried out at 25°C for 12 hours. GC central control reaction process: GC central control monitors that the peak integral area of raw materials on the GC spectrum / (integrated area of raw material peak + product peak integral area) < 0.5%, that is, the GC central control monitors that the remaining amount of reaction raw materials < 0.5%, to end the reaction, the raw material here refers to 1,4-cyclohexanedione monoethylene glycol ketal, and the product here refers...
Embodiment 3
[0071] (一)8-氟-1,4-二氧杂螺[4.5]癸-7-烯的合成
[0072] 依次将312g二氯甲烷,DBU(456.6g,3.0mol,3.0eq),1,4-环己二酮单乙二 醇缩酮(156.2g,1.0mol,1.0eq)加入1000ml的三口反应瓶中。
[0073] 开启搅拌,降温至10±5℃,滴加DAST(193.4g,1.2mol,1.2eq),控制内温在 10±5℃,滴加完毕后,每小时升温5℃,升至25℃,并在25℃反应12小时。GC 中控反应进程。当GC中控监测到在GC谱图上原料出峰积分面积 / (原料出峰积分 面积+产物出峰积分面积)<0.5%时,即GC中控监测到反应原料剩余量<0.5%, 结束反应,这里的原料是指1,4-环己二酮单乙二醇缩酮,这里的产物是指8-氟-1,4- 二氧杂螺[4.5]癸-7-烯。
[0074] 反应结束后,将反应液缓慢加入750g冰水中,分出有机相,水相用312g二氯 甲烷萃取三次(二氯甲烷总用量为312g×3),合并全部有机相,用盐酸(750g, 1mol / L)洗涤数次,GC中控有机相无DBU残留,然后有机相用750g饱和碳酸氢 钠洗涤数次,直至pH值为7~8,再用无水硫酸钠干燥后过滤,将所得有机相滤液 减压浓缩得到粗品,粗品减压精馏,当温度升至80℃的时候开始接收馏分,得到 产品145g。
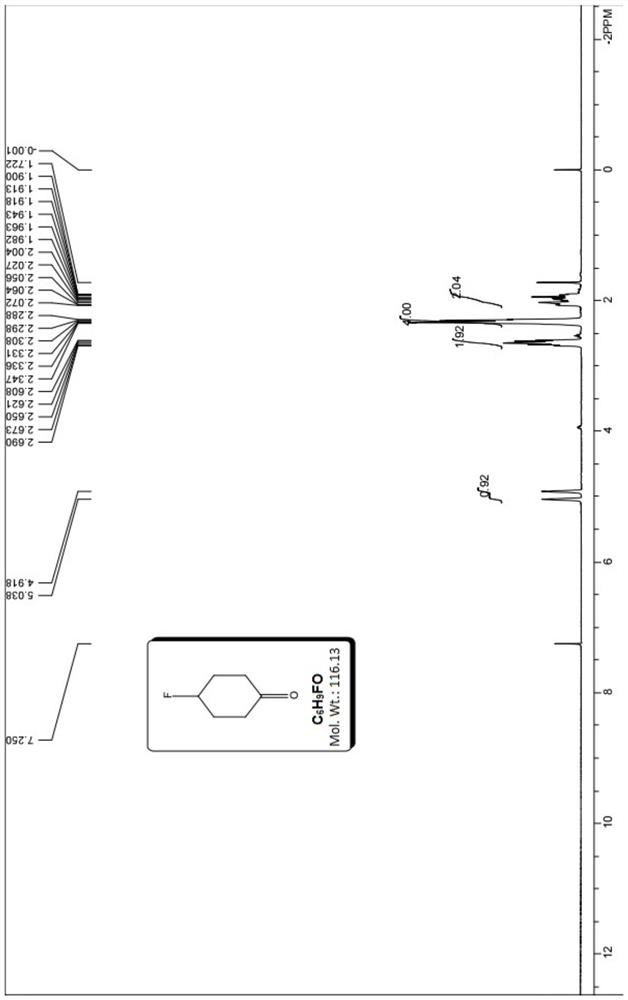
[0075] 对所得产品进行气相色谱(GC)和核磁共振氢谱(HNMR)检测,以确定其 结构式和纯度。由核磁共振氢谱谱图可以确定所得产品为8-氟-1,4-二氧杂螺[4.5] 癸-7-烯,GC测得纯度98.2%,计算收率为92%。
[0076] (二)8-氟-1,4-二氧杂螺[4.5]癸烷的合成
[0077] Same as Example 1.
[0078] (三)4-氟环己酮的合成
[0079] Same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com