A kind of acid-base indicator and its preparation method and application
A technology of acid-base indicator and preparation steps, which is applied in material analysis through observation of the influence on chemical indicators, analysis through chemical reaction of materials, organic chemistry, etc., to achieve simple synthesis, good stability, and color development sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 thymol phthalein
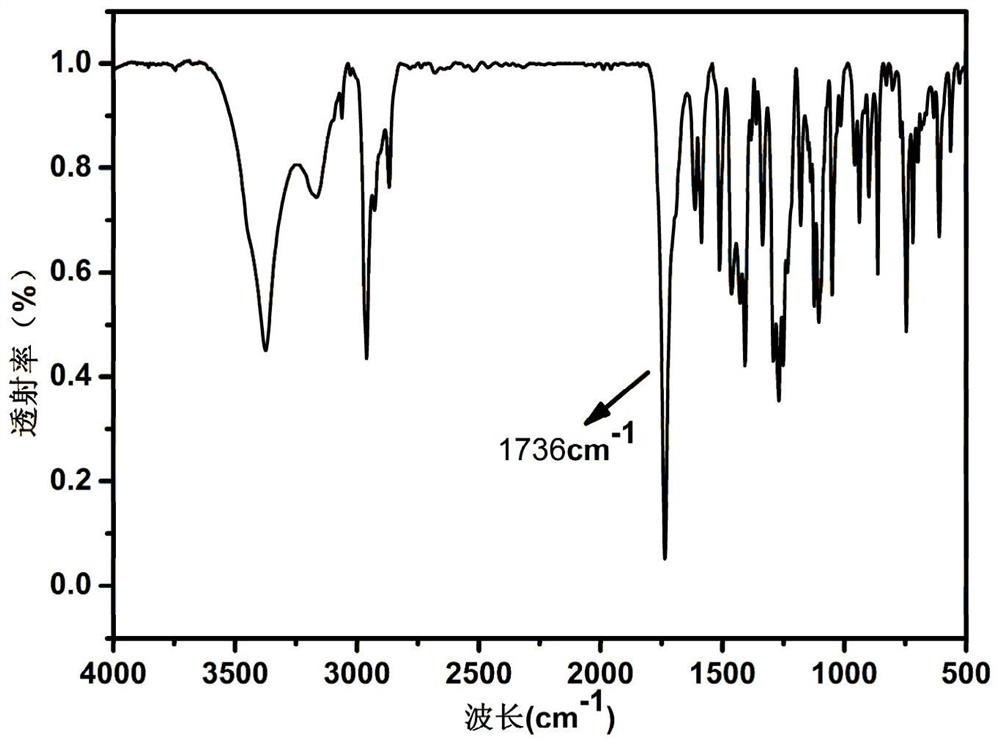
[0026] Mix 2.0mmol thymolphthalein and 2.0mmol urotropine (HMTA) into a 50ml three-neck flask, add 10ml (0.15mol) trifluoroacetic acid (TFA) under ice-bath conditions, and stir the solution for 30min before warming up to room temperature. Continue to heat the oil bath to 72°C for reflux reaction for 24h. After the reaction is stopped, excess TFA is removed by rotary evaporation. Add 50ml H 2 O, the stirred solution was heated to 60°C, filtered after 30 min, and dried to obtain a solid. The solid was further separated by column chromatography, using petroleum ether and dichloromethane at a ratio of 1:3 (v / v) as a developer, and finally dried by rotary evaporation to obtain a light yellow powder.
Embodiment 2
[0027] The preparation of embodiment 2 thymol phthalein
[0028] Mix 1.5mmol thymolphthalein and 2.0mmol urotropine (HMTA) into a 50ml three-neck flask, add 15ml (0.2mol) trifluoroacetic acid (TFA) under ice-bath conditions, and stir the solution for 30min before warming up to room temperature. Continue to heat the oil bath to 72°C for reflux reaction for 8h. After the reaction is stopped, excess TFA is removed by rotary evaporation. Add 30ml H 2 O, the stirred solution was heated to 60°C, filtered after 30 min, and dried to obtain a solid. The solid was further separated by column chromatography, using petroleum ether and dichloromethane at a ratio of 1:3 (v / v) as a developer, and finally dried by rotary evaporation to obtain a light yellow powder.
Embodiment 3
[0029] The preparation of embodiment 3 thymol phthalein
[0030] Mix 1.5mmol thymolphthalein and 1.0mmol urotropine (HMTA) into a 50ml three-necked flask, add 15ml (0.2mol) trifluoroacetic acid (TFA) under ice-bath conditions, and stir the solution for 30min before warming up to room temperature. Continue to heat the oil bath to 72° C. for reflux reaction for 12 h. After the reaction is stopped, excess TFA is removed by rotary evaporation. Add 50ml H 2 O, the stirred solution was heated to 60°C, filtered after 30 min, and dried to obtain a solid. The solid was further separated by column chromatography, using petroleum ether and dichloromethane at a ratio of 1:3 (v / v) as a developer, and finally dried by rotary evaporation to obtain a light yellow powder.
[0031] figure 1 is the color change of thymol phthalein in buffer solution at pH 1-14 (from left to right). figure 2 It is the color change diagram of thymol phthalein in pH buffer solution of 10.0, 10.2, 10.4, 10.6, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com