Anti-tumor protein peptide capable of inhibiting FOXM1
A protein peptide and anti-tumor technology, applied in the fields of genetic engineering and oncology, can solve the problem of inability to obtain induced pluripotent stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
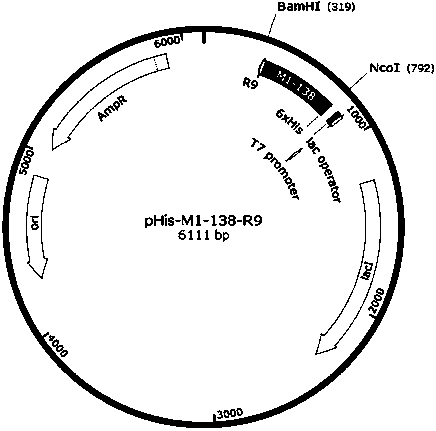
[0035] Example 1, Construction of prokaryotic expression vector pHis-M1-138-R9.
[0036] 1. Amplification of the His-M1-138-R9 gene fragment. Design primers to amplify the gene fragment by PCR. The primer sequence is: Primer 1 (upstream primer): GCG CCC ATG GTG CAT CAC CAT CAC CAT CAC ATG AAA ACT AGC CCC CGTCG, primer 2 (downstream primer): GCG GGA TCC CTA CCT TCT CCT TCT CCT TCT CCT TCT CCT CAGGGT CAC TTC TGT C. Using pcDNA3.1-FOXM1 as a template, under the guidance of primers 1 and 2, the reaction system for PCR amplification of M1-138 was as follows: clone plasmid pcDNA3.1-FOXM1 (80ng / μL) 1 μL, 10X PCR Buffer (ThermoScientific) 5 μL, dNTPs (2mM each) 5μL, MgSO 4 Solution (25mM) 4μL, KOD-Plus-Neo (1.0U / μL) 1μL, primer 1 (100μM) 1μL, primer 2 (100μM) 1μL, DMSO 2μL, add deionized water to supplement the reaction system to 50μL. PCR reaction conditions: first 94°C for 5min; then 95°C for 30sec, 57°C for 30sec, 68°C for 50sec, a total of 30 cycles; then 68°C for 10min, 4°C fo...
Embodiment 2
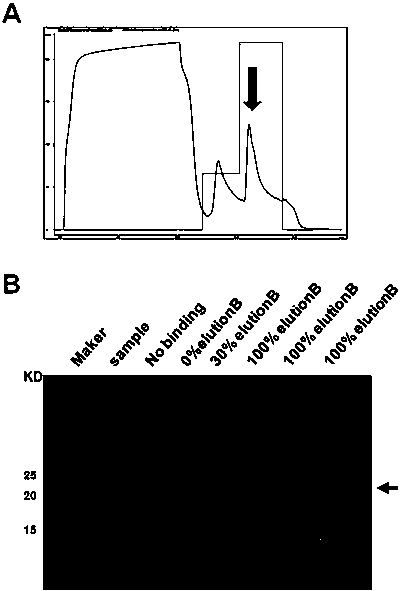
[0039] Example 2, large-scale purified M1-138 recombinant protein.
[0040] 1. Induced expression of M1-138 recombinant protein. The prokaryotic expression vector pHis-M1-138-R9 was transformed into Escherichia coli Rostta DE3 competent cells, cultured at 37°C overnight (12-16hr), randomly selected single clones, and inoculated into 5mL LB medium (containing 25μg / mL ampicillin and 25μg / mL chloramphenicol), shake culture at 37℃ for 6-8hr. Add the bacteria solution to 100 mL of LB culture solution (containing 25 μg / mL ampicillin and 25 μg / mL chloramphenicol) and shake at 37°C overnight (12-16hr), take the bacteria solution to detect the OD 600 value, adjust OD 600 When the value reached 0.8-1, IPTG inducer (final concentration 8 μM) was added, and shaking culture was induced at 20°C for 20 hr. Centrifuge at 4000rpm for 20min to collect the bacteria, and use 15mL Binding Buffer (20mM Na 3 PO 4 , 500mM NaCl, 20mMimidazole, pH 7.4) to resuspend the bacteria, and sonicate for 4...
Embodiment 3
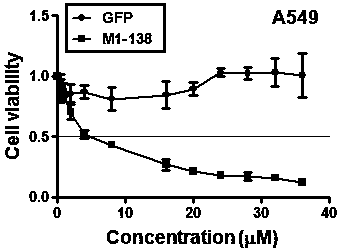
[0042] Example 3, M1-138 recombinant protein inhibits lung cancer cell line A549.
[0043] In order to verify the inhibitory effect of M1-138 on the proliferation of lung cancer cells, lung cancer A549 cells were selected, and the commercial Cell Counting Kit (CCK) kit was used to investigate the effect of M1-138 on the proliferation of tumor cells. Seed the cell suspension (100 μL, 4000 cells / well) in a 96-well plate. Place the culture plate in the incubator (37°C, 5% CO 2) after pre-incubation for 12 hr, the cells were treated with different concentrations of M1-138 (0.5, 1, 2, 4, 8, 16, 20, 24, 28, 32, 36 μM) and cultured for another 12 hr. Add 10 μL of CCK WST-8 reagent to each well, incubate in the incubator for 1-4hr, measure the absorbance at 450nm with a microplate reader, and calculate the cell viability of the cells to be tested, cell viability*(%)=[A( Added drug)-A (blank)] / [A (no drug added)-A (blank)] ×100, where, A (medicated): absorbance of cells, CCK solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com