Recombinant escherichia coli for heterologously synthesizing ambrein and construction method thereof
A technology for recombining Escherichia coli and ambroxol, which is applied in the biological field and can solve problems such as the inability to meet the requirements of industrial production, low yield, and no reports of ambroxol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
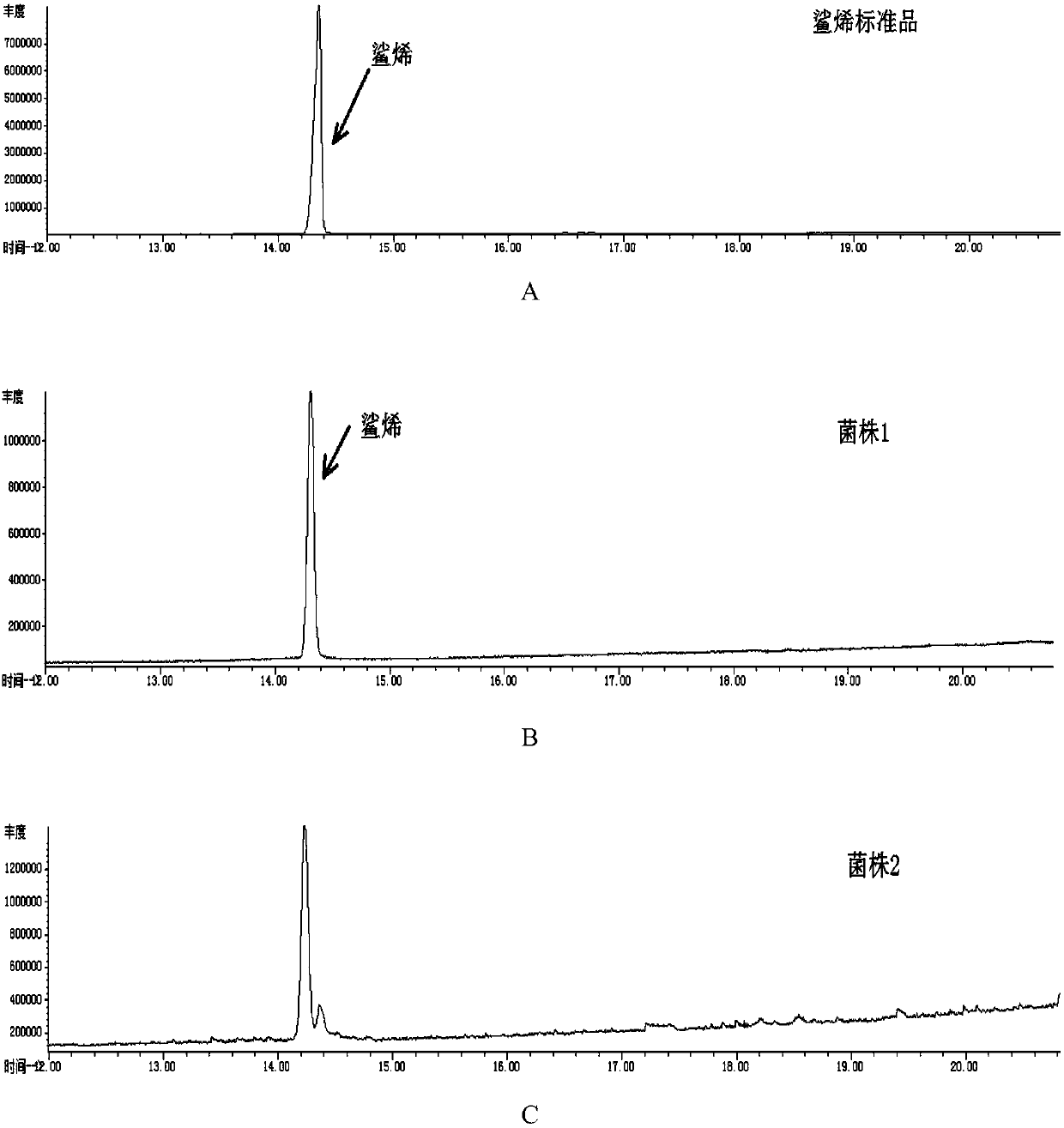
[0051] The construction of the recombinant escherichia coli of embodiment 1 synthetic squalene
[0052] In order to construct the squalene synthetic metabolic pathway on the Escherichia coli genome, the Saccharomyces cerevisiae squalene synthase gene ERG9, which was truncated by 26 amino acid residues at the C-terminus, was first constructed downstream of the constitutive trc promoter as an operator module trcp-ERG9 .
[0053] The upstream and downstream homology arms of the Saccharomyces cerevisiae squalene synthase gene ERG9 truncated by 26 amino acid residues at the C-terminal were fused with the lacZ gene site of Escherichia coli ATCC.47076 (hereinafter referred to as E. coli ATCC.47076) as Donor DNA;
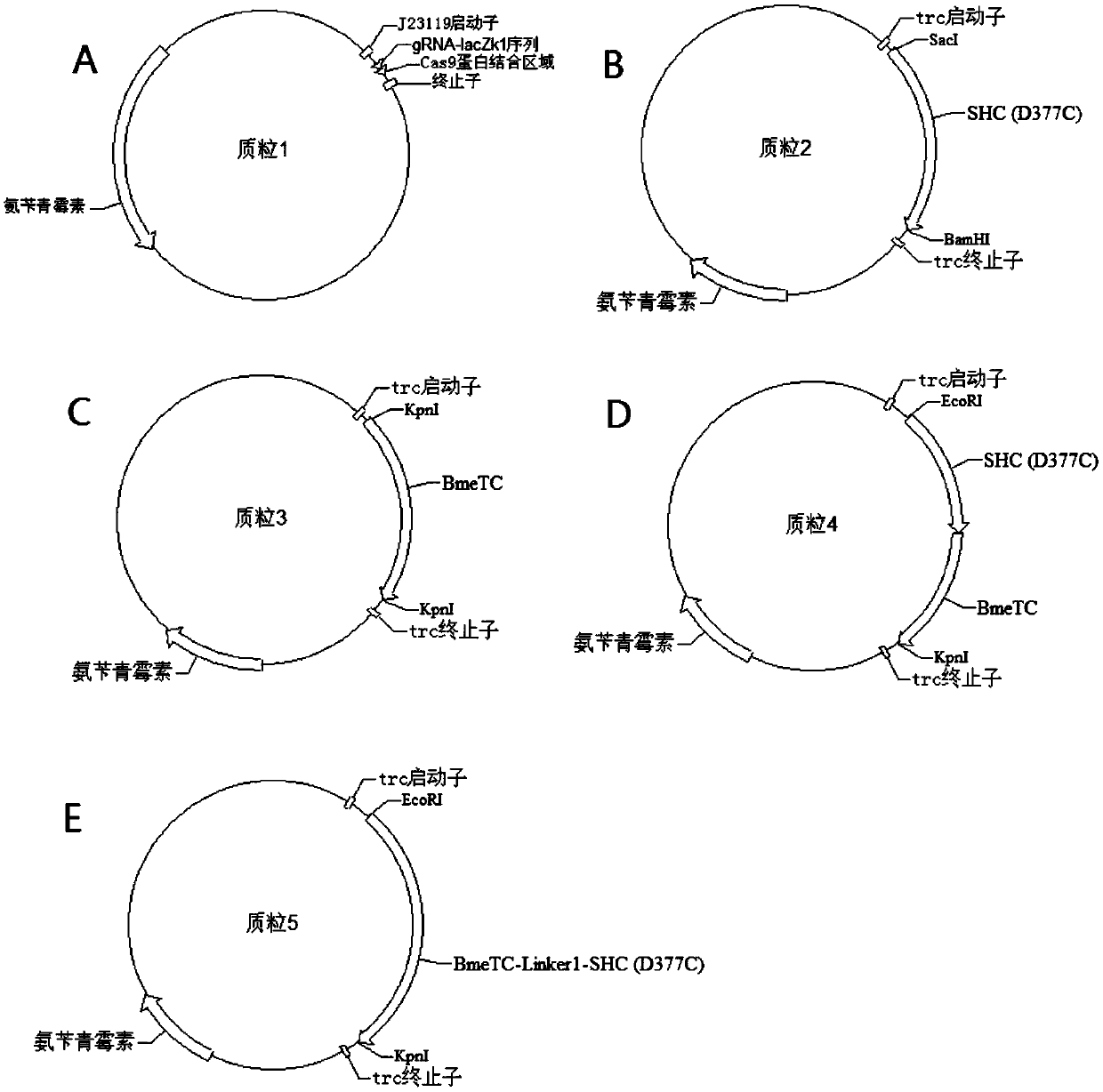
[0054] The expression plasmid for constructing the gRNA targeting the lacZ gene locus is plasmid 1.
[0055] The donor DNA and plasmid 1 were transformed into E. coli ATCC.47076, and the S. cerevisiae squalene synthase gene ERG9, which was truncated by 26 amino acid resid...
Embodiment 2
[0064] Example 2 Construction method of recombinant Escherichia coli for heterologous synthesis of ambroxol
[0065] 1. Construction of expression vectors for enzymes related to ambroxol synthesis
[0066] ① According to the amino acid residue sequence of Alicyclobacillus acidocaldarius squalene-hopene cyclase after the 377th amino acid residue was mutated to cysteine residue, codon optimization for Escherichia coli was carried out, by Kings Rui Biological Technology Co., Ltd. designed and synthesized Alicyclobacillus acidocaldarius squalene-hopene cyclase gene D377C SHC (SEQ ID NO.2) after mutating the 377th amino acid residue to cysteine residue .
[0067] The D377C SHC gene fragment was inserted into the SacI and BamHI restriction sites of the Escherichia coli expression plasmid p5C (Zhenquan Lin et al., Microbial Cell Factories. 2014, 13(1):104) to obtain plasmid 2.
[0068] The specific steps are: using the D377C SHC gene sequence (SEQ ID NO.2) as a template, and us...
Embodiment 3
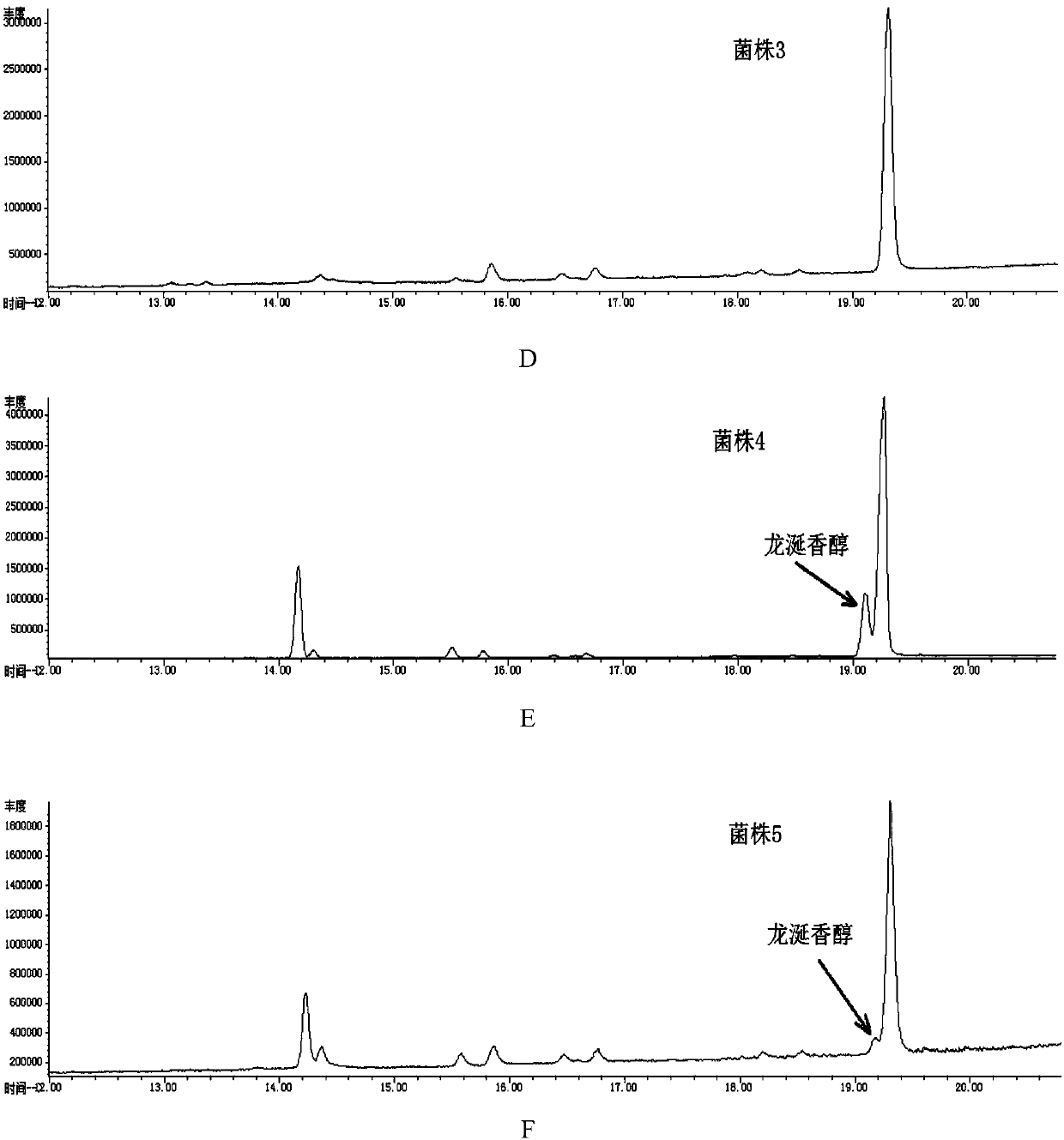
[0080] Example 3 Construction Method of the Second Recombinant Escherichia coli for Heterologous Synthesis of Ambroxol
[0081]1. Construction of the expression vector of the BmeTC-Linker1-D377C SHC fusion protein for the synthesis of ambroxol
[0082] Design of fusion protein expressed by fusion of Alicyclobacillus acidocaldarius squalene-hopene cyclase and Bacillus megaterium tetraprenyl-β-curcumene cyclase after mutating the 377th amino acid residue to cysteine residue Gene (BmeTC-Linker1-D377C SHC). The BmeTC-Linker1-D377C SHC gene (SEQ ID NO.4) was inserted into the EcoRI and KpnI restriction sites of the Escherichia coli expression plasmid p5C to obtain plasmid 5.
[0083] The design principle of the fusion protein is: delete the stop codon of the BmeTC gene, connect it with the amino acid sequence of the connecting peptide (Linker1), and then connect it with the D377C SHC gene fragment with the stop codon. Due to the absence of the stop codon of the BmeTC nucleotide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com