Preparation and application of phospholipid complex as EV71 and CAV16 virus inhibitor
A CAV16, phospholipid complex technology, applied in the direction of antiviral agents, drug delivery, medical preparations of non-active ingredients, etc., can solve the problems of clinical application and dosage form development restrictions, restrictions on oral absorption rate, etc., to improve in vivo absorption capacity , Easy to prepare, fast absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of EV71 virus and CAV16 virus inhibitor (TJAB-1099) phospholipid complex
[0025] Weigh 0.10 g of TJAB-1099 raw material, 0.36 g of egg yolk lecithin, add 5 mL of chloroform, and react at 40°C for 3 hours until clarification. The chloroform was volatilized by rotary evaporation under reduced pressure to dryness, and vacuum-dried for 24 hours to obtain 0.46 g of phospholipid complex. It was sealed and packaged, and stored in a refrigerator at 4°C.
Embodiment 2
[0026] Embodiment 2: Preparation of EV71 virus and CAV16 virus inhibitor (TJAB-1099) phospholipid complex
[0027] Weigh 0.10 g of TJAB-1099 raw material, 0.36 g of egg yolk lecithin, add 5 mL of dichloromethane, and react at 40°C for 0.5 hours until clarification. Dichloromethane was volatilized by rotary evaporation under reduced pressure to dryness, and vacuum-dried for 24 hours to obtain 0.46 g of phospholipid complex, which was sealed and packaged, and stored in a refrigerator at 4°C.
Embodiment 3
[0028] Embodiment 3: the preparation of EV71 virus and CAV16 virus inhibitor (TJAB-1099) phospholipid complex
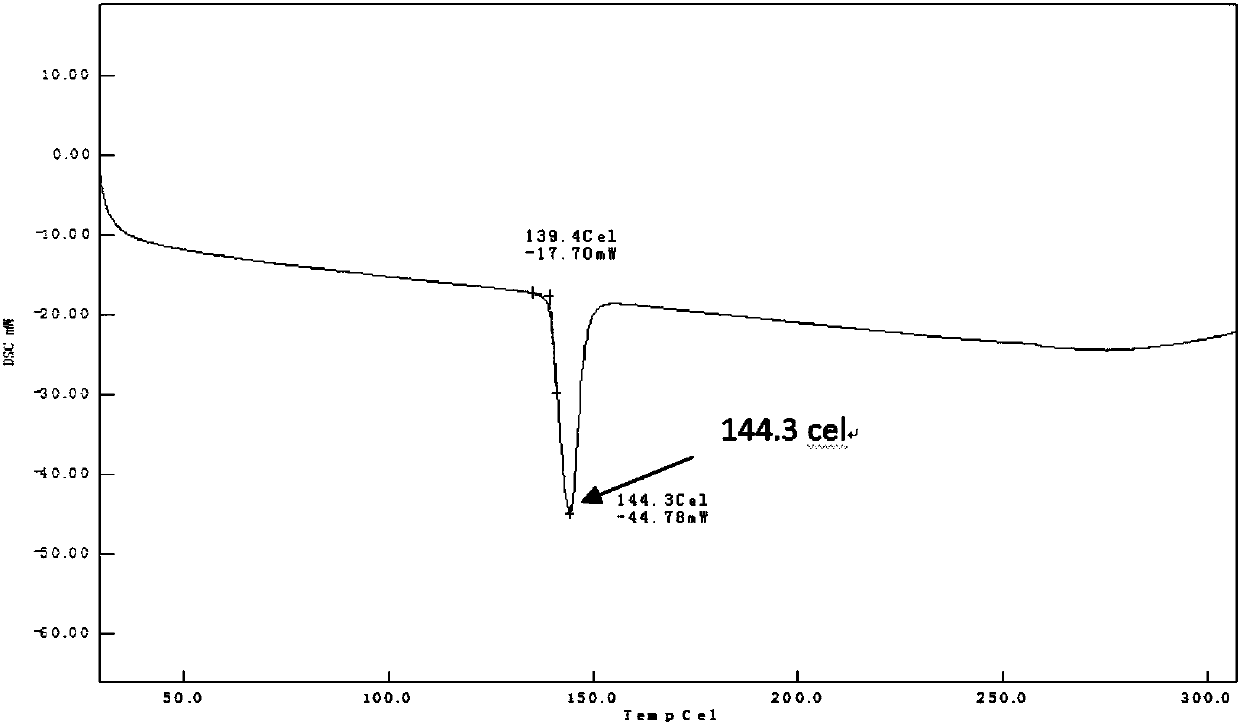
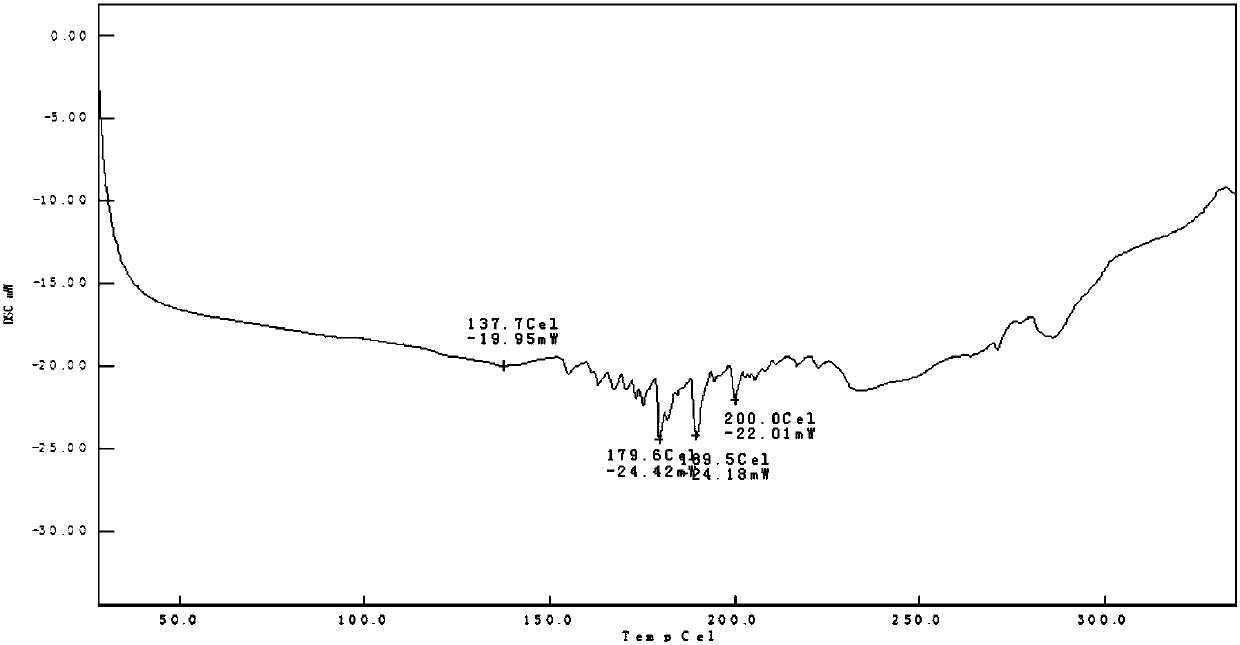
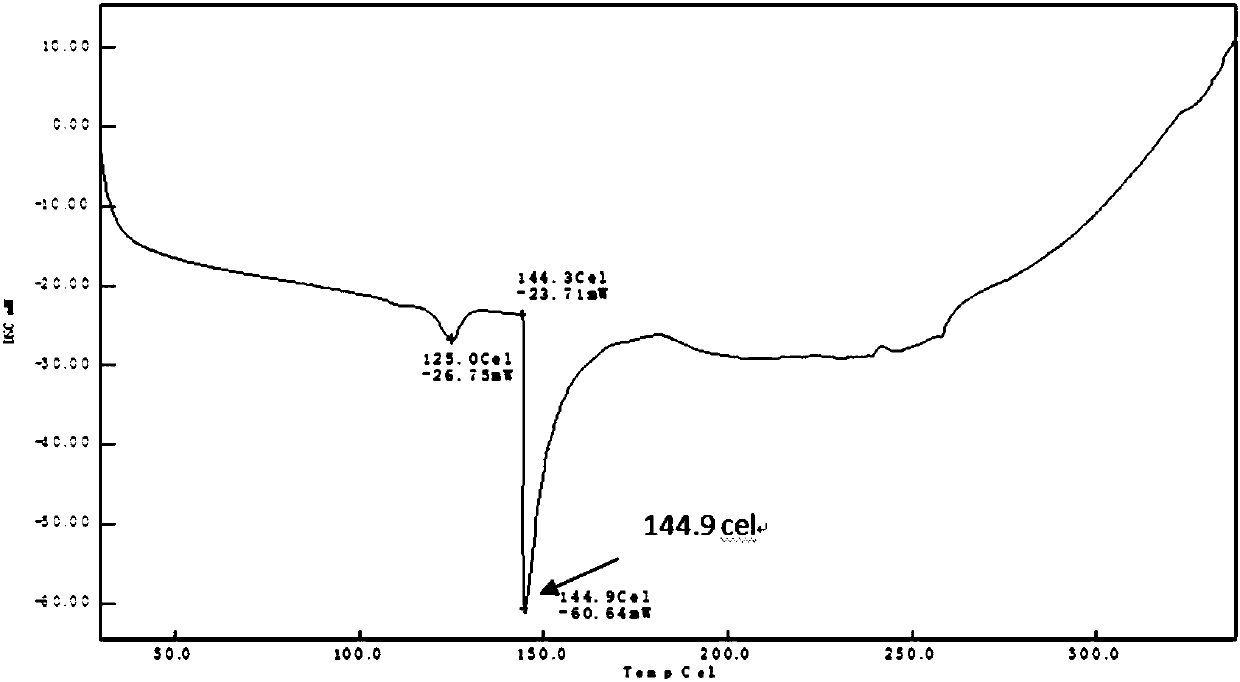
[0029] Weigh 0.10 g of TJAB-1099 raw material, 0.36 g of egg yolk lecithin, add 5 mL of cyclohexane, and react at 60°C for 3 hours until clarification. Dichloromethane was volatilized by rotary evaporation under reduced pressure to dryness, and vacuum-dried for 24 hours to obtain 0.46 g of phospholipid complex, which was sealed and packaged, and stored in a refrigerator at 4°C. The formation of the complex was confirmed by thermal analysis and nuclear magnetic resonance, as shown in Figure 1A to Figure 2B shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com