Synthesis method of benzoic acid under illumination condition
A technology of light conditions and synthesis methods, applied in the chemical industry, can solve problems such as high energy consumption, serious pollution, and unacceptable benzoic acid, and achieve the effects of reducing energy consumption, production costs, and catalyst costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
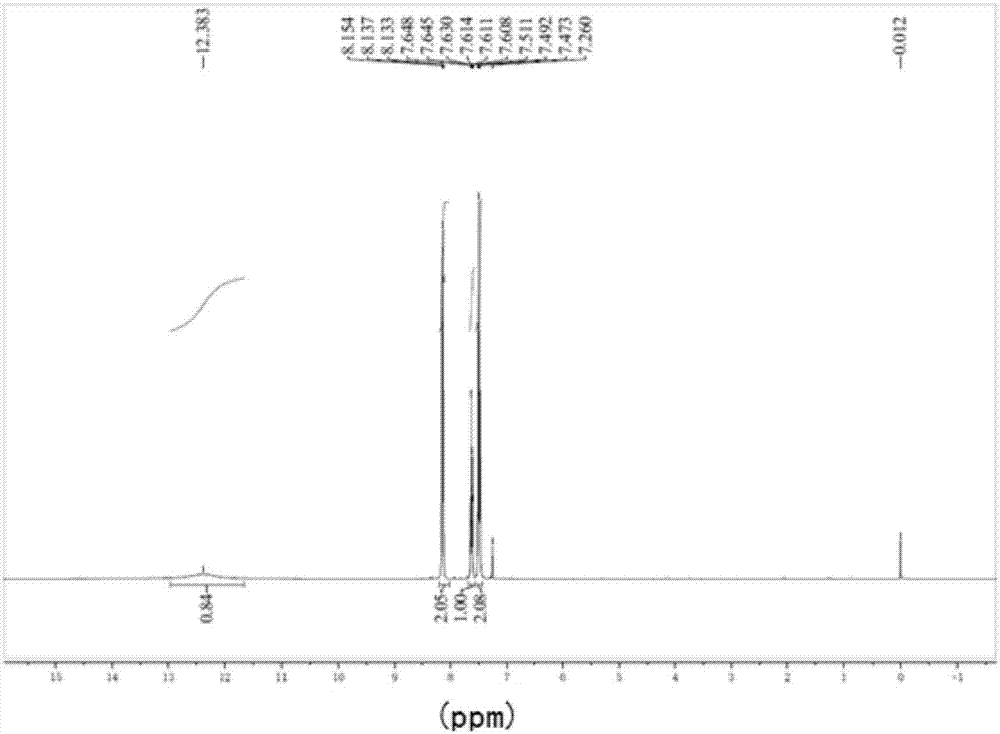
[0025] Add 10 kg of toluene and 2% mass fraction of photocatalyst 2-hydroxybenzophenone into a 50 L reactor, stir in the air atmosphere, and react under the light irradiation of two 1500 W xenon lamps at 25 °C for 48 h. After the reaction time was up, through high-performance liquid chromatography analysis, only a single product benzoic acid was generated, and the conversion rate of toluene exceeded 99%. Add aqueous sodium hydroxide solution to the reaction mixture until the pH is neutral, extract and recover the photosensitizer with ethyl acetate, add dilute hydrochloric acid to the aqueous layer to adjust the pH to 1-2, recrystallize, dry in vacuum, NMR identification, the product quality is 13.0 kg, The yield was 98%.
Embodiment 2
[0027] Add 10 kg of toluene and 3% mass fraction of photocatalyst 2-hydroxybenzophenone into a 50 L reactor, stir in the air atmosphere, and react under the light irradiation of two 1500 W xenon lamps for 46 hours at a temperature of 25 °C . After the reaction time was up, through high-performance liquid chromatography analysis, only a single product benzoic acid was generated, and the conversion rate of toluene exceeded 99%. Add aqueous sodium hydroxide solution to the reaction mixture until the pH is neutral, extract and recover the photosensitizer with ethyl acetate, add dilute hydrochloric acid to the aqueous layer to adjust to PH = 1-2, recrystallize, vacuum dry, NMR identification, the product quality is 13.1kg, The yield was 99%.
Embodiment 3
[0029] Add 10kg of toluene and 4% mass fraction of photocatalyst 2-hydroxybenzophenone into a 50 L reactor, stir in the air atmosphere, and react under the light irradiation of two 1500 W xenon lamps for 45 hours at a temperature of 25 °C . After the reaction time was up, through high-performance liquid chromatography analysis, only a single product benzoic acid was generated, and the conversion rate of toluene exceeded 99%. Add aqueous sodium hydroxide solution to the reaction mixture until the pH is neutral, extract and recover the photosensitizer with ethyl acetate, add dilute hydrochloric acid to the aqueous layer to adjust to pH = 1-2, recrystallize, vacuum dry, NMR identification, the product quality is 13.0 kg, The yield was 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com