Preparation method of high-conductivity lithium ion solid-state electrolyte
A solid electrolyte and solid electrolyte technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve problems such as poor environmental stability, and achieve the effects of improving conductivity, reducing energy, and increasing lithium ion concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
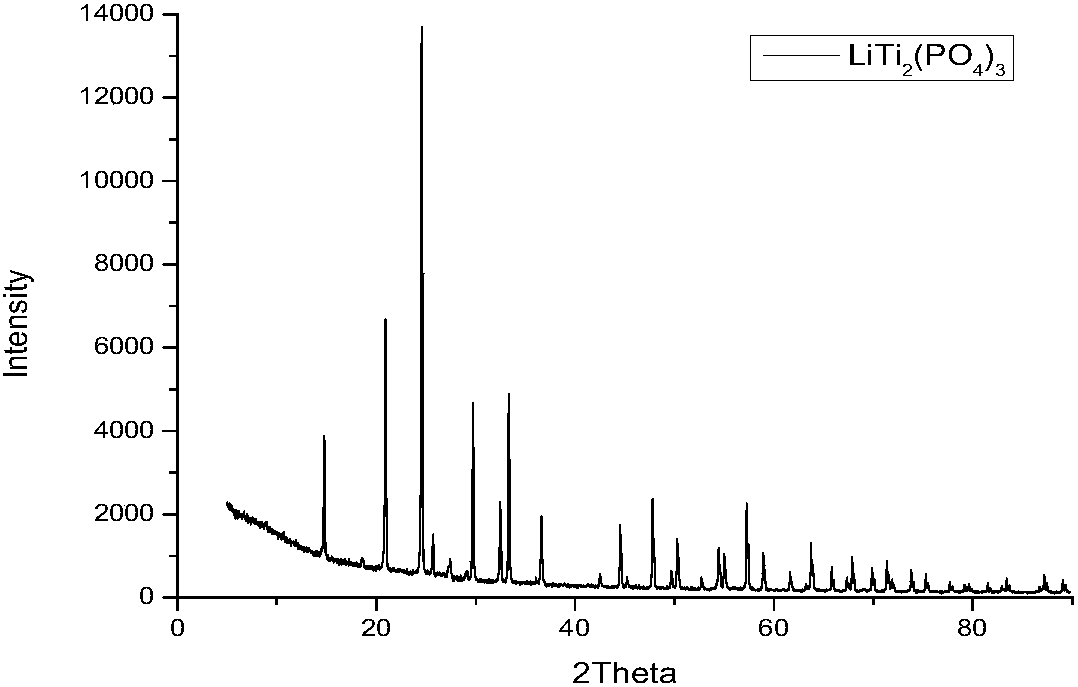
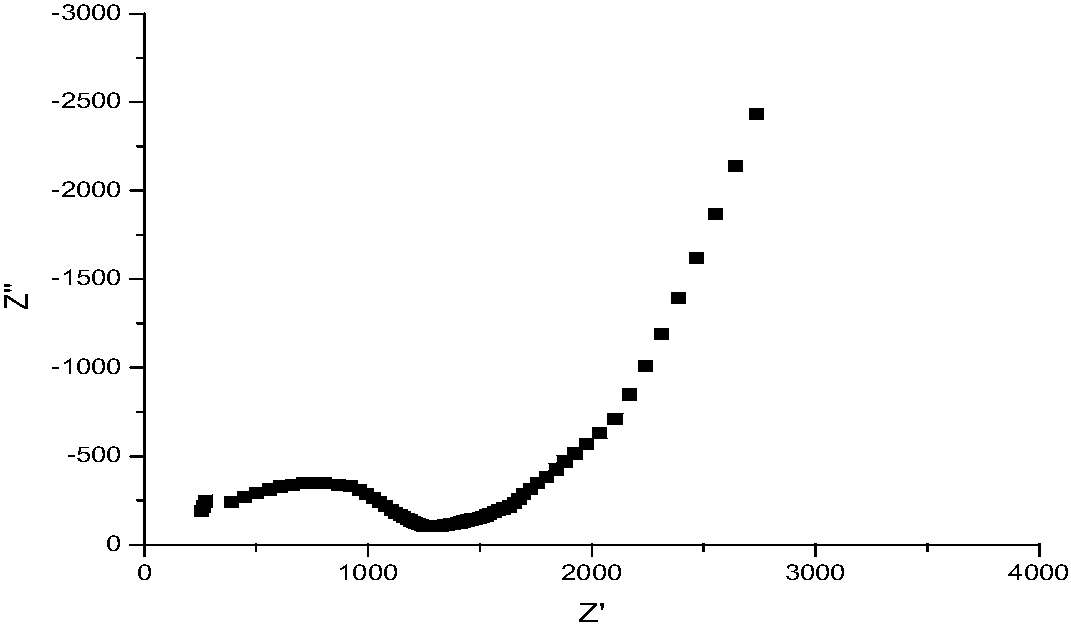
[0024] Put the raw CH 3 COOLi, Al(NO 3 ) 3 ·9H 2 O, Ti (OC 4 H 9 ) 4 , NH 4 H 2 PO 4 Weighing according to the molar ratio of 1.3:0.3:1.7:3, add the raw materials to the reaction beaker in turn, and keep stirring at room temperature to form a light yellow slurry; slowly add ammonia water to the reaction beaker, and at the same time add a small amount of deionized water, pH = 12; then the reaction beaker was magnetically stirred in a water bath at 90°C for 0.5h to form a uniform, stable and layered white precipitate. Transfer the stable and layered white precipitate to a crucible and place it in a blast drying oven, heat at 150 °C for 10 h and dry to obtain a white precursor; the precursor is heated in air at a heating rate of 5 °C / min to 800 °C, and kept at 800°C for 12h to obtain white powder. First grind manually for 5 minutes, and then use a planetary ball mill for 6 hours at a speed of 200 r / min. The grinding medium is zirconia spheres, and the mass ratio of the ...
Embodiment 2
[0026] Put the raw CH 3 COOLi, Al(NO 3 ) 3 ·9H 2 O, Ti (OC 4 H 9 ) 4 , NH 4 H 2 PO 4 Weighing according to the molar ratio of 1.5:0.3:1.7:3, add the raw materials into the reaction beaker in turn, and keep stirring at room temperature to form a light yellow slurry; slowly add ammonia water to the reaction beaker, and at the same time add a small amount of deionized water, pH = 12; then the reaction beaker was magnetically stirred in a water bath at 95°C for 1.5h to form a uniform and stable layered white precipitate. Transfer the stably stratified white precipitate to a crucible and place it in a blast drying oven, heat at 150 °C for 14 h and dry to obtain a white precursor; the precursor is heated to 750 °C at a heating rate of 5 °C / min in air, And kept at 750℃ for 10h to obtain white powder. First grind manually for 5 minutes, and then use a planetary ball mill for 6 hours at a speed of 200 r / min. The grinding medium is zirconia spheres, and the mass ratio of the s...
Embodiment 3
[0028] Put the raw CH 3 COOLi, Al(NO 3 ) 3 ·9H 2 O, Ti (OC 4 H 9 ) 4 , NH 4 H 2 PO 4Weighing according to the molar ratio of 1.4:0.3:1.7:3, add the raw materials into the reaction beaker in turn, and keep stirring at room temperature to form a pale yellow slurry; slowly add ammonia water to the reaction beaker, and at the same time add a small amount of deionized water, pH = 12; then the reaction beaker was magnetically stirred in a water bath at 80°C for 1 h to form a uniform, stable and layered white precipitate. Transfer the stably stratified white precipitate to a crucible and place it in a blast drying oven, heat at 150 °C for 14 h and dry to obtain a white precursor; the precursor is heated to 650 °C at a heating rate of 5 °C / min in air, and kept at 650°C for 8h to obtain white powder. First grind manually for 5 minutes, and then use a planetary ball mill for 6 hours at a speed of 200 r / min. The grinding medium is zirconia spheres, and the mass ratio of the sph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com