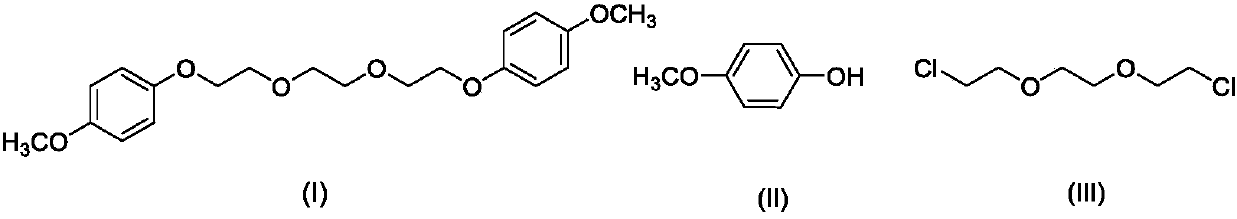
Synthesis method of 1,2-di(2-(4-dimethoxyphenoxy) ethoxy) ethane
A technology of dimethoxyphenoxy and methoxyphenoxy, which is applied in the field 1, can solve the problems of complex post-processing and long reaction time, and achieve the effect of simple post-processing and increasing reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
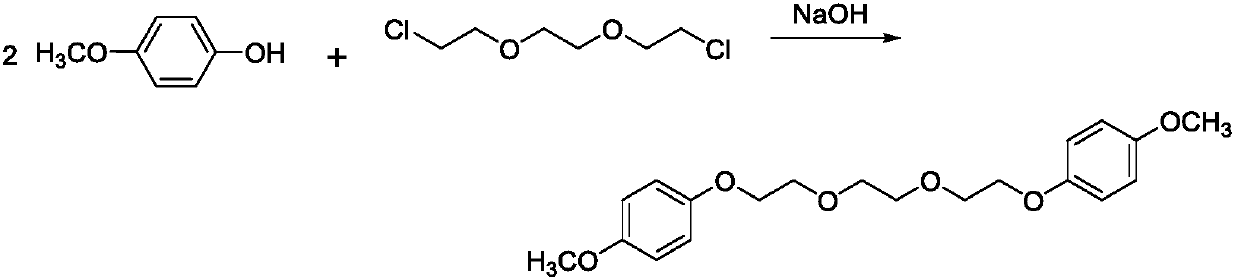
[0025] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 9.30g (50mmol) of 1,2-(dichloroethane)ethane were added to 100ml of N,N-dimethylformamide, Add 6.72g (120mmol) of potassium hydroxide to it, react at 150°C for 12h, pour the reaction liquid into water to obtain brown turbid liquid 1;
[0026] Cool the above brown turbid liquid 1 to -15°C for 4h, filter the solution to obtain a white flake solid, add ether to the white flake solid, heat to 35°C, filter out insolubles, cool to -15°C, keep for 4h , The solution was filtered to obtain 17.92 g of white powdery solid 1,2-bis(2-(4-dimethoxyphenoxy)ethoxy)ethane. The yield is 98.35%, the purity is 98.11%, and the melting point is 67-68°C.
[0027] Structure Identification:
[0028] IR(KBr)ν:3444,3057,3014,2959,2931,2902,2871,2838,2786,2700,2590,2545,
[0029] 2474,2345,2125,2050,1986,1905,1866,1740,1626,1509,1453,1385,1332,1287,1250,
[0030] 1229,1189,1132,1116,1072,1055,1031,948,927,883,821,731,593,522,...
Embodiment 2
[0035] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 9.30g (50mmol) of 1,2-(dichloroethane)ethane were added to 100ml of N,N-dimethylformamide, 6.72g (120mmol) of potassium hydroxide was added thereto, reacted at 130°C for 12h, and the reaction solution was poured into water to obtain brown turbid liquid 1;
[0036] Cool the above brown turbid liquid 1 to -15°C for 4h, filter the solution to obtain a white flake solid, add ether to the white flake solid, heat to 35°C, filter out insolubles, cool to -15°C, keep for 4h , The solution was filtered to obtain 17.63 g of white powdery solid 1,2-bis(2-(4-dimethoxyphenoxy)ethoxy)ethane. The yield is 96.76%, the purity is 98.06%, and the melting point is 67-68°C.
Embodiment 3
[0038] Under stirring at a temperature of 20°C, 13.66g (110mmol) of 4-methoxyphenol and 9.30g (50mmol) of 1,2-(dichloroethane)ethane were added to 100ml of N,N-dimethylformamide, 6.16g (110mmol) of potassium hydroxide was added thereto, reacted at 150°C for 12h, and the reaction solution was poured into water to obtain brown turbid liquid 1;
[0039] Cool the above brown turbid liquid 1 to -15°C for 4h, filter the solution to obtain a white flake solid, add ether to the white flake solid, heat to 35°C, filter out insolubles, cool to -15°C, keep for 4h , The solution was filtered to obtain 17.25 g of white powdery solid 1,2-bis(2-(4-dimethoxyphenoxy)ethoxy)ethane. The yield is 94.68%, the purity is 98.13%, and the melting point is 67-68°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com