Method for separating nucleic acids from fepe tissue
A tissue and nucleic acid technology, applied in the field of nucleic acid isolation to achieve high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1: Isolation of Genomic DNA (Including Removal of Paraffin) from FFPE Tissue Samples
[0070] (1) FFPE block fragments and paraffin removal
[0071] Cut the FFPE block to a thickness of 6 μm, then place one or two FFPE tissue segments in a 1.5 mL tube. Add 1 mL of xylene and mix, then centrifuge at 12,000 x g for 3 min at room temperature. After removing the supernatant, 0.8 mL of xylene and 0.4 mL of EtOH were added and mixed, centrifuged at 12000×g for 3 minutes at room temperature, and the supernatant was removed. Add 1 mL of EtOH and mix again, centrifuge at 12,000 x g for 3 min at room temperature, and remove the supernatant. In order to completely evaporate the remaining EtOH, incubation was performed at room temperature for 0.5 h to 1 h.
[0072] (2) Cleavage and Genomic DNA Isolation
[0073] Add 90 μL of lysis buffer (10 mM Tris-Cl, pH 8.0, 100 mM EDTA, pH 8.0, 0.5% SDS) and 10 μL of proteinase K to the tube containing the paraffin-depleted FFPE tis...
Embodiment 2
[0077] Example 2: Isolation of Genomic DNA from FFPE Tissue Samples (without Paraffin Removal)
[0078] (1) FFPE block fragmentation
[0079] Cut the FFPE block to a thickness of 6 μm, then place one or two FFPE tissue segments in a 1.5 mL tube.
[0080] (2) Cleavage and Genomic DNA Isolation
[0081] Add 200 μL of lysis buffer (10 mM Tris-Cl, pH 8.0, 100 mM EDTA, pH 8.0, 0.5% SDS), 200 μL of mineral oil, and 10 μL of proteinase K to the tube containing the FFPE tissue fragment and mix, then incubate at 56 °C Incubate overnight.
[0082] Next, the lower phase was separated and transferred to a new tube. 100 μL magnetic beads (AMPure XP) and 100 μL solution A (2.5M NaCl, 20% PEG-8000) were added to the tube and mixed. Tubes were then incubated at room temperature for 3 minutes. Tubes were incubated on a magnetic stand for 5 minutes and the supernatant removed.
[0083] After adding 500 μL of washing buffer (85% EtOH) and reacting, the ethanol supernatant was removed on a ...
Embodiment 3
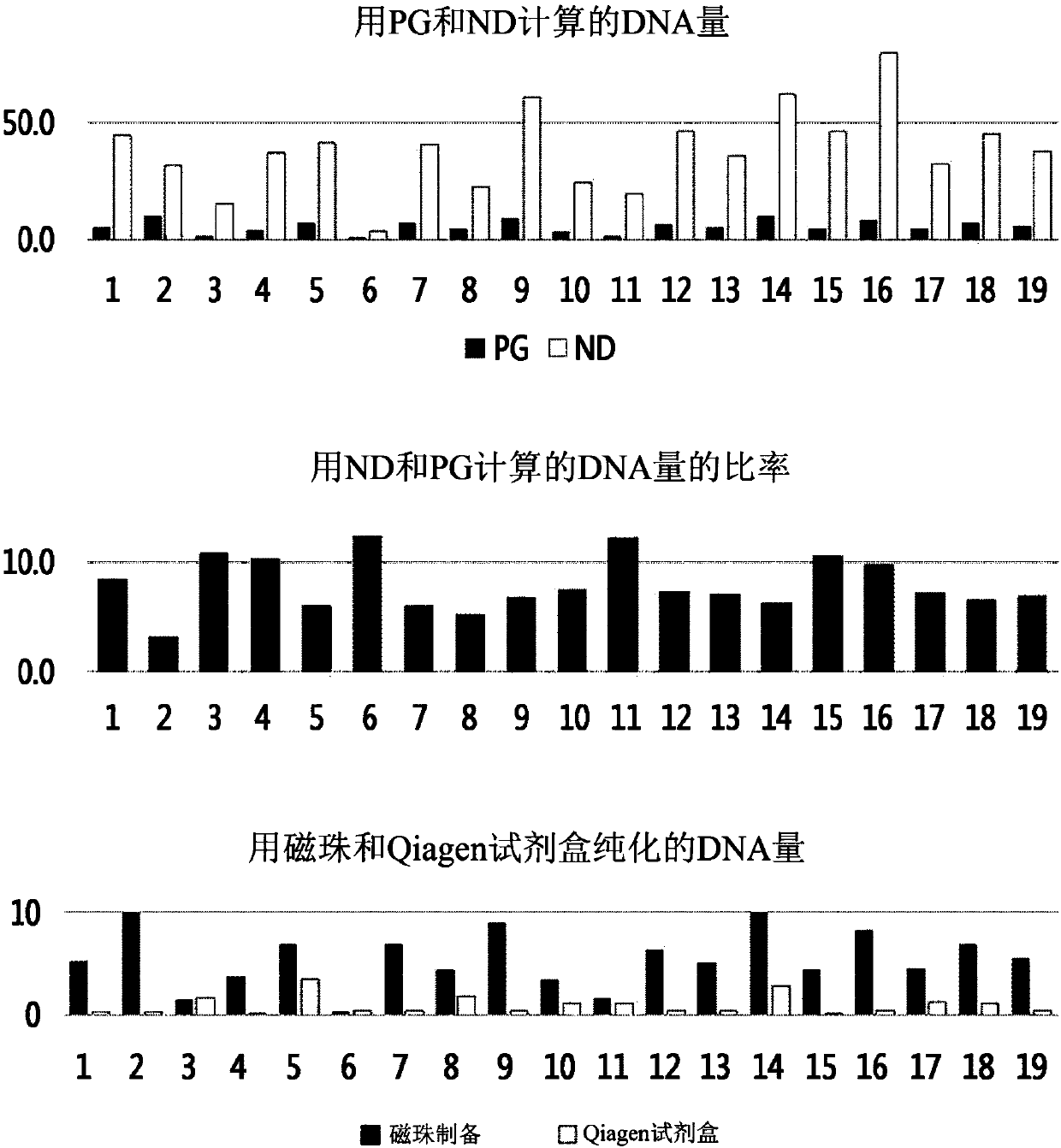
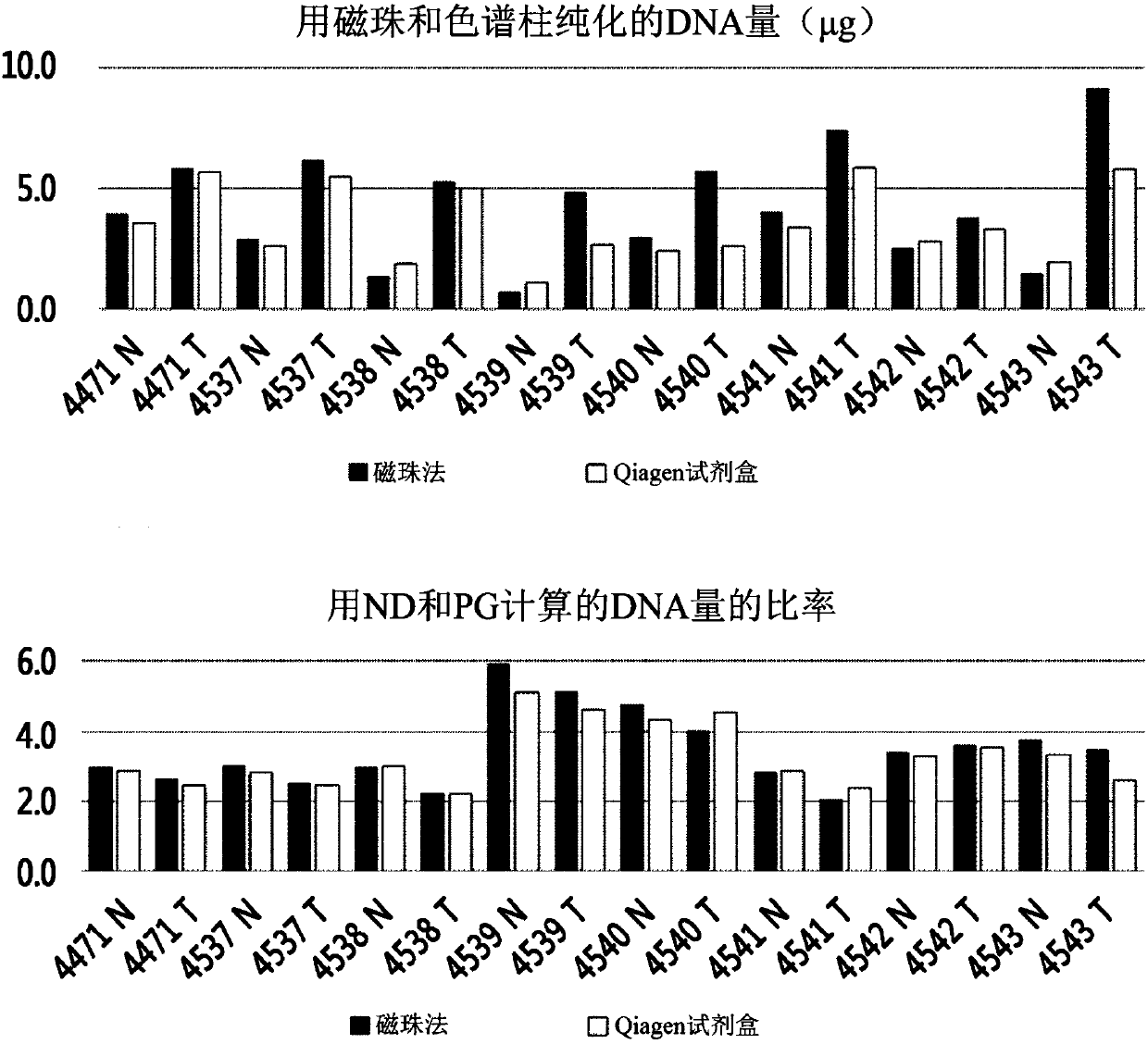
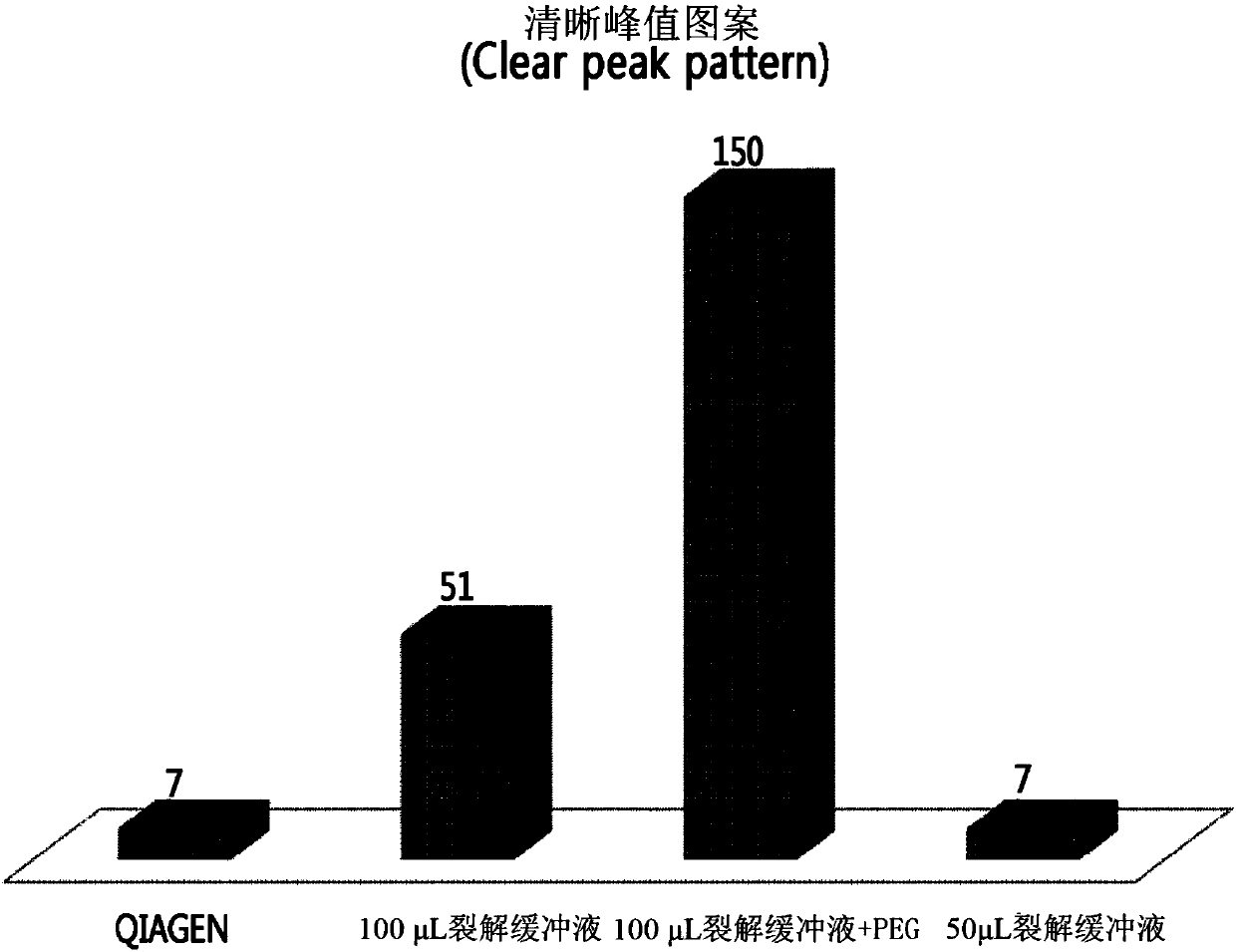
[0086] Embodiment 3: Quantitative analysis of DNA
[0087] (1) Quantification using NanoDrop (ND)
[0088] NanoDrop 2000 (Thermo Scientific) was used in the dsDNA concentration measurement mode. After measuring the blank value using the DNA extraction eluate as a reference, use 1 μL of the extracted DNA to measure the OD 260 Under the absorbance, the concentration was calculated using the following formula: dsDNA concentration = absorbance (OD 260 )*50ng / μL. Calculate the final yield by multiplying by 100 μL (total volume of extraction solvent).
[0089] (2) Quantification using PicoGreen (PG)
[0090] Quant-Ti PicoGreen dsDNA assay kit (Invitrogen) was purchased for measuring dsDNA concentration.
[0091] A, the preparation of PicoGreen reagent
[0092] Thirty minutes before measuring the concentration, dilute the PicoGreen reagent to 1 / 200 with 1×TE buffer, wrap it in aluminum foil and store it in the dark at room temperature.
[0093] B. Preparation of standard conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com