A kind of triazole Schiff base compound and its preparation method and application
A technology for triazole Schiff bases and compounds is applied in the field of triazole Schiff base compounds and their preparation, which can solve the problems of increasing bacterial infection rate and morbidity rate, and achieve easy implementation, good application prospect, broad-spectrum antibacterial active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 5-(4-(2-Hydroxy-benzyl)amino-5-methyl-4H-1,2,4-triazole-3-sulfanyl)-1,3,4-oxadiazole-2 -The preparation of mercaptan, step is as follows:
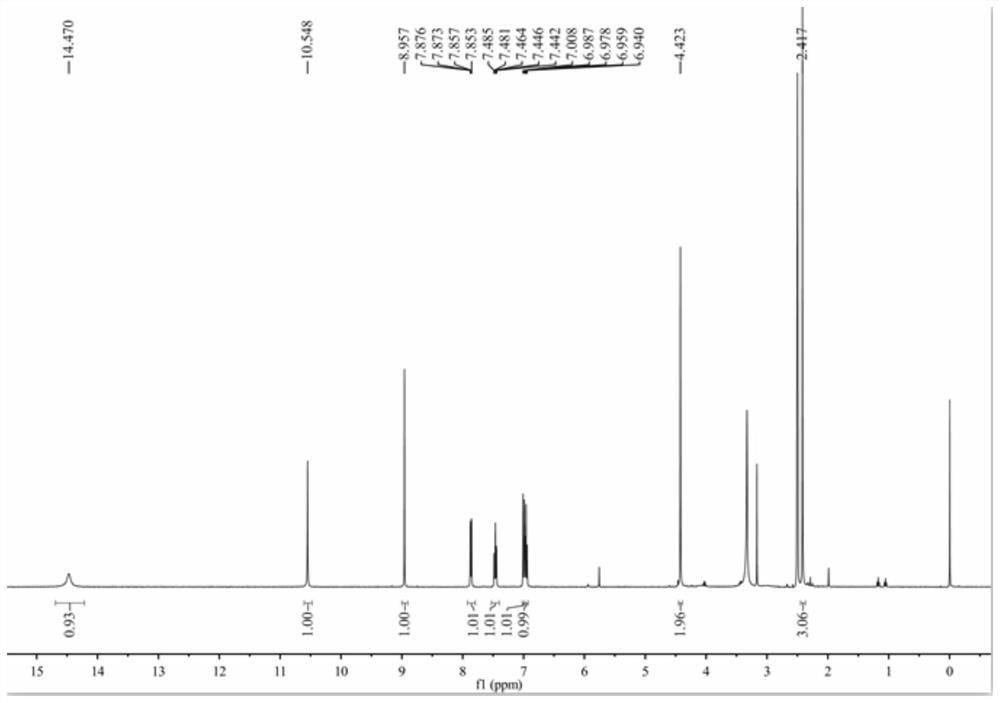
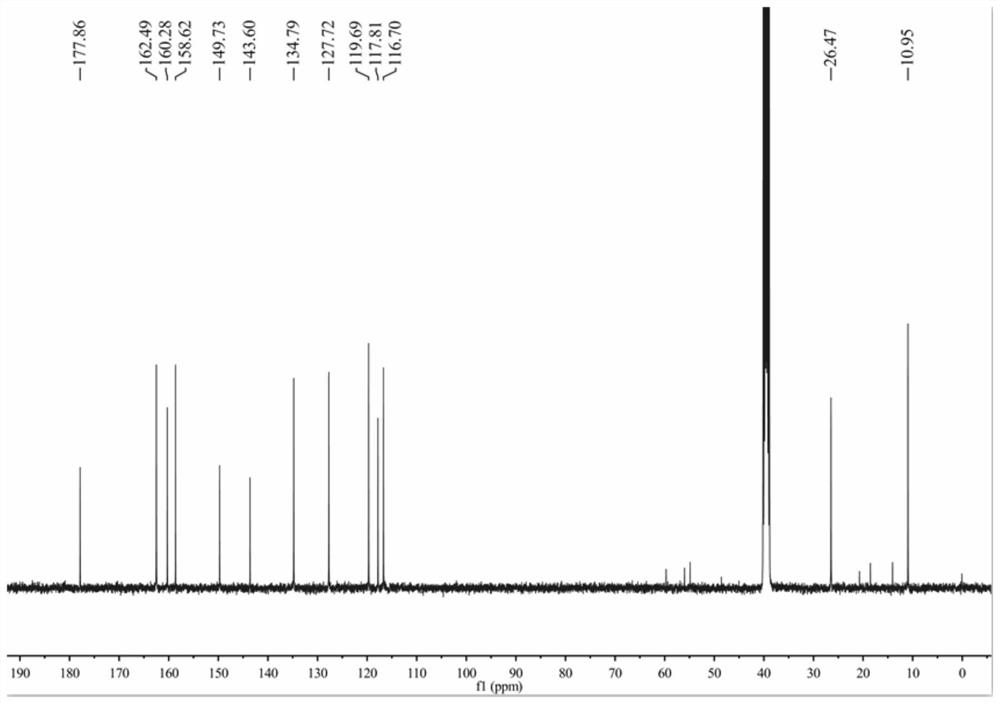
[0048] 1) Add 20mL of methanol into a 50mL three-necked flask, and add 2.16g (10mmol) of 3-methyl-4-amino-5-ethoxycarbonylmethylthio-1,2,4-triazole under stirring to make it Dissolve completely, slowly add 1.2mL (30mmol) of hydrazine hydrate dropwise, follow the reaction with thin-layer chromatography (TLC), the developer is a mixture of dichloromethane and methanol with a volume ratio of 6:1, after the reaction is completed, spin off part of the solvent, and crystallized to obtain 1.6g of 3-methyl-4-amino-5-acetic acid hydrazide with a yield of 78.8%. m.p.146-148℃. 1 H NMR (400MHz, DMSO-d 6 ),9.30(s,1H,NH),5.88(s,2H,NH 2 ),4.31(s,2H,NH 2 ),3.76(s,2H,CH 2 ),2.28(s,3H,CH 3 ). 13 C NMR (100MHz, DMSO-d 6 ),167.20,153.80,151.01,33.87,10.24.ESI-MS,m / z:203.0720[M+H] + .
[0049] 2) Add 15mL of ethanol to a 100mL three-necked fl...
Embodiment 2
[0053] 5-(4-(2-Hydroxy-5-bromo-benzyl)amino-5-methyl-4H-1,2,4-triazole-3-sulfanyl)-1,3,4-oxa The preparation steps of oxadiazole-2-thiol are as follows:
[0054] 1) The preparation method of 3-methyl-4-amino-5-acetic acid hydrazide is the same as in Example 1.
[0055] 2) Preparation method and implementation of 5-(4-amino-5-phenyl-4H-1,2,4-triazole-3-sulfanyl)-1,3,4-oxadiazole-2-thiol Example 1 is the same.
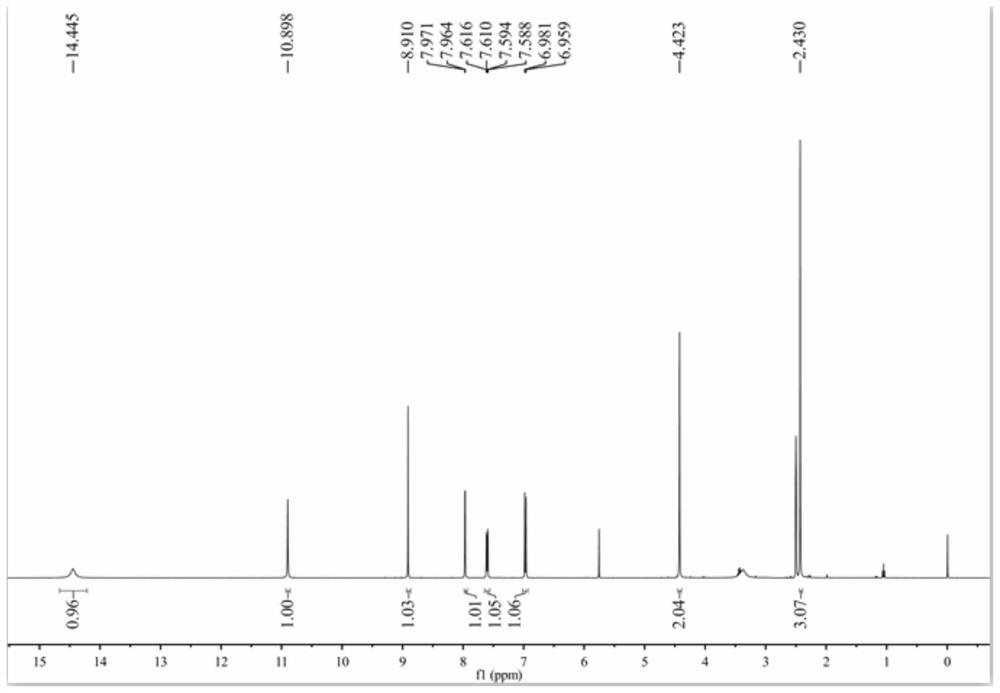
[0056] 3) Add 20mL of ethanol to a 50mL round bottom flask, slowly add 2.44g (10mmol) of 5-(4-amino-5-phenyl-4H-1,2,4-triazole-3-sulfanyl) -1,3,4-Oxadiazole-2-thiol was stirred and dissolved, and then 2.21g (1.1mmol) of 5-bromosalicylaldehyde was added, and the pH was adjusted to 5-6 with dilute hydrochloric acid, and the reaction was stirred at 78°C. Thin-layer chromatography (TLC), the developer is a mixture of dichloromethane and methanol with a volume ratio of 10:1. After the raw material point disappears, the reaction solution is concentrated and separated by sil...
Embodiment 3
[0059] 5-(4-(2-Hydroxy-5-chloro-benzyl)amino-5-methyl-4H-1,2,4-triazole-3-sulfanyl)-1,3,4-oxa The preparation steps of oxadiazole-2-thiol are as follows:
[0060] 1) The preparation method of 3-methyl-4-amino-5-acetic acid hydrazide is the same as in Example 1.
[0061] 2) Preparation method and implementation of 5-(4-amino-5-phenyl-4H-1,2,4-triazole-3-sulfanyl)-1,3,4-oxadiazole-2-thiol Example 1 is the same.
[0062] 3) Add 20mL of ethanol to a 50mL round bottom flask, slowly add 2.44g (10mmol) of 5-(4-amino-5-phenyl-4H-1,2,4-triazole-3-sulfanyl) -1,3,4-Oxadiazole-2-thiol was stirred and dissolved, then 1.71g (1.1mmol) 5-chlorosalicylaldehyde was added, the pH was adjusted to 5-6 with dilute hydrochloric acid, the reaction was stirred at 78°C, and Thin-layer chromatography (TLC), the developer is a mixture of dichloromethane and methanol with a volume ratio of 10:1. After the raw material point disappears, the reaction solution is concentrated and separated by silica gel c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com