Recombinant vaccine and application thereof
A technology of vaccines and recombinant vectors, applied in vaccines, applications, recombinant DNA technology, etc., can solve the problems of sPD1 synergistic anti-tumor effect disappearing, no anti-tumor effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
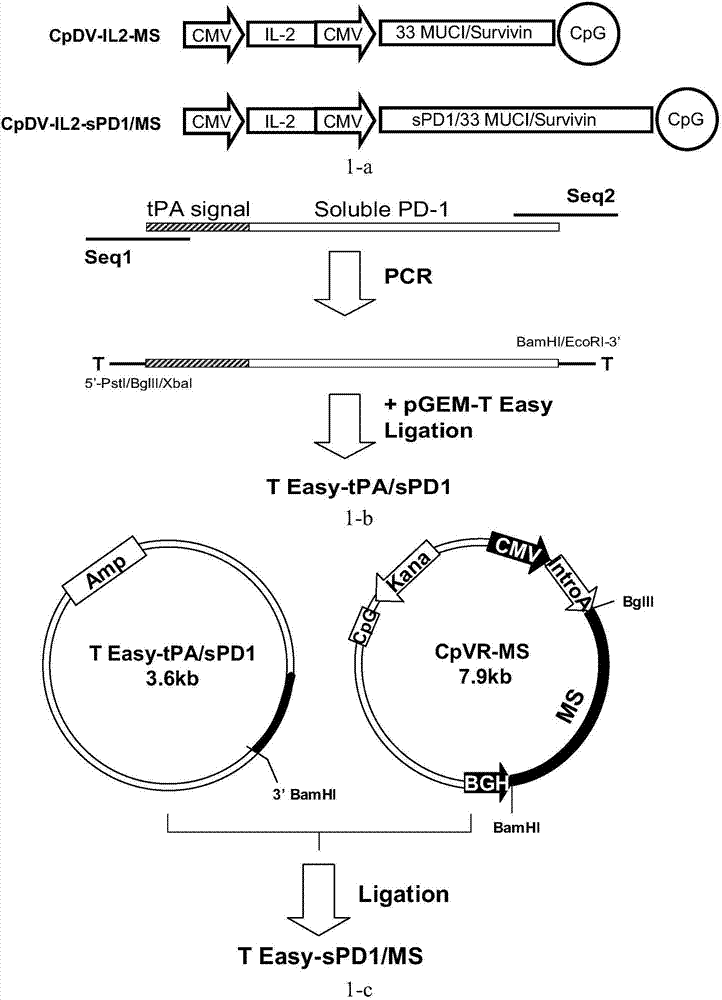
[0130] Embodiment 1: Construction of recombinant DNA vector vaccine sPD1 / MS
[0131] 1.1 tPA / sPD1 gene synthesis and T Easy-tPA / sPD1 construction
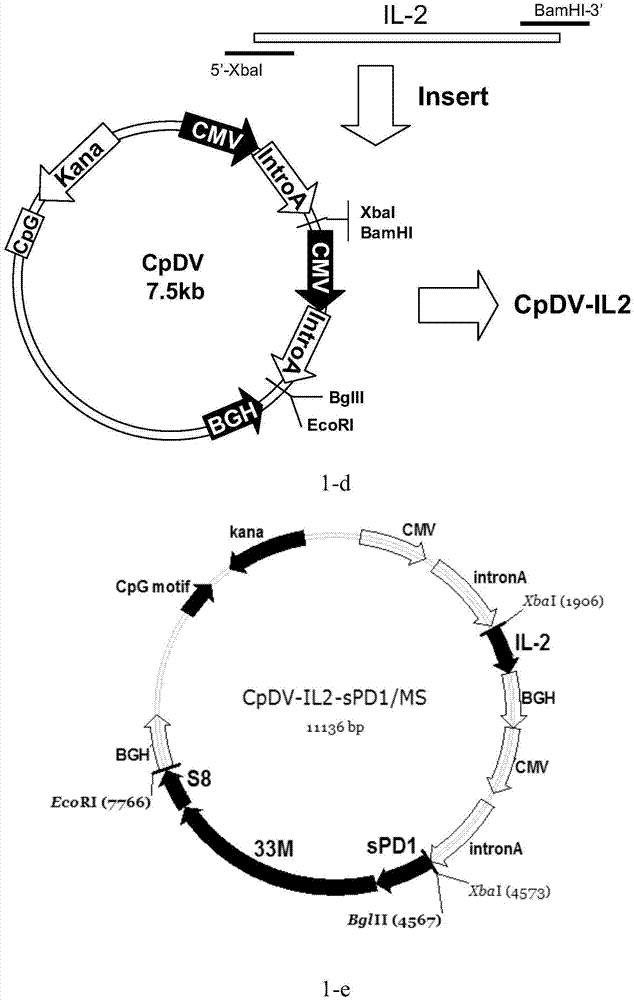
[0132] According to GeneBank number NM_005018.2 gene synthesis of human PD-1 extracellular segment sequence, the 5' end of the gene sequence is connected with a 69bp tPA signal peptide sequence (see SEQ ID NO: 10), the purpose of introducing the tPA signal peptide is to improve the fusion protein The ability of sPD1 / MS to secrete and express extracellularly. Thereafter, PCR technology was used to introduce restriction sites at both ends of the tPA / sPD1 gene, and the PCR reaction system was 50 μl, including 0.1 μg of the tPA / sPD1 DNA template described in SEQ ID NO: 1, primers SEQ ID NO: 12 and SEQ ID NO: 13 Final concentration 20pmol, 5μl Ex Taq Buffer (10×), 4μl dNTP Mixture (2.5mM each), 1.25U ExTaq enzyme (Takara); 35 cycle amplification reactions. Subsequently, the PCR product recovered from the gel was connected to the pGEM...
Embodiment 2
[0141] Embodiment 2: Preparation of recombinant protein vaccine sPD1 / MS
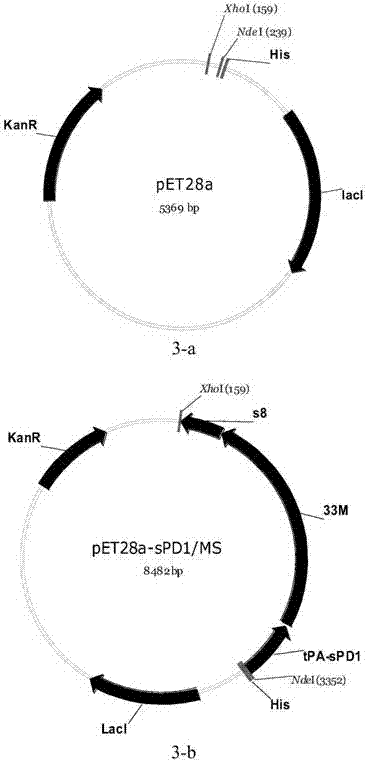
[0142] Protein vaccines are another common form of tumor vaccines. Different from DNA vaccines or virus vector vaccines, protein vaccines enter cells in the form of antigenic proteins and can directly activate dendritic cells. At the same time, because the protein form cannot be integrated into the host cell genome, it has Higher security. In this example, the prokaryotic expression vector pET28a-sPD1 / MS was constructed to induce the expression of the fusion protein vaccine (sPD1 / MS). The specific construction process is as follows:
[0143] The prokaryotic expression plasmid pET28a-sPD1 / MS was constructed by homologous recombination: the pET28a plasmid (see image 3 -a) After double digestion with Nde I and Xho I, a 5289 bp fragment of the vector sequence was recovered from the gel. The sequence of the sPD1 / MS cistron in CpDV-IL2-sPD1 / MS was amplified by PCR, and the 5' end of the primer had a sequenc...
Embodiment 3
[0148] Embodiment 3: Construction of recombinant viral vector vaccine sPD1 / MS
[0149] 3.1 Obtaining of recombinant poxvirus vaccine rMVA-sPD1 / MS
[0150] The application of Modified Vaccinia Ankara (MVA) co-carried with tumor antigens for immunotherapy of tumors is relatively safe. The shuttle plasmid of MVA is pSC11, which has multiple cloning restriction sites that can be inserted into foreign genes. In this example, the sPD1 gene was inserted into the previously constructed plasmid pSC11-MS (see the invention patent with the patent number ZL200910252427.X), To construct pSC11-sPD1 / MS, the thymidine kinase left and right arms (TKL, TKR) on the vector can be homologously recombined with MVA, and at the same time, the blue spot screening of recombinant MVA (rMVA) can be carried out through the lacZ gene on the vector.
[0151] The specific method for constructing the pSC11-sPD1 / MS shuttle plasmid is first to carry out a PCR reaction with primers SEQ ID NO: 16 and SEQ ID NO: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com