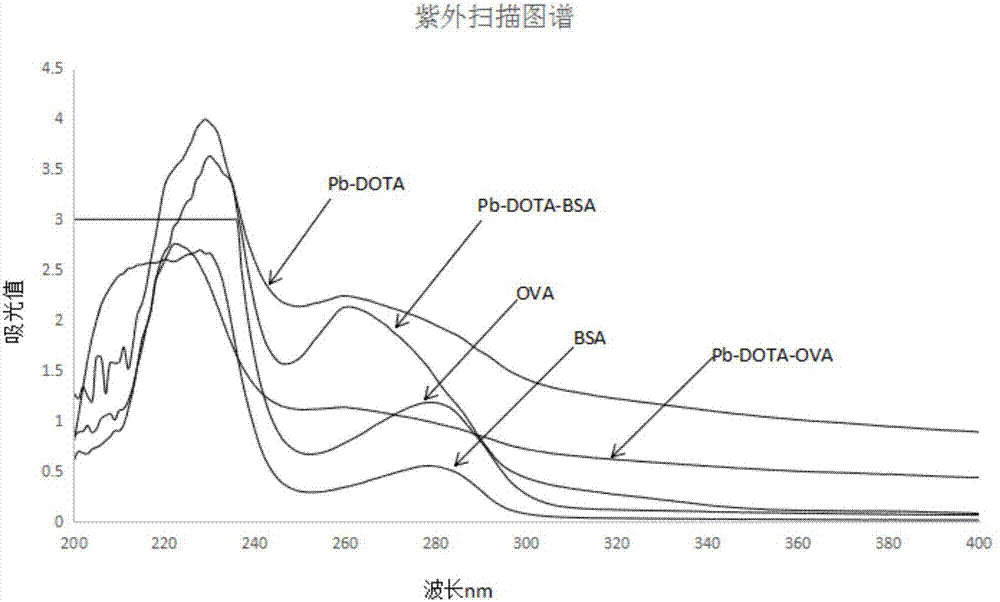
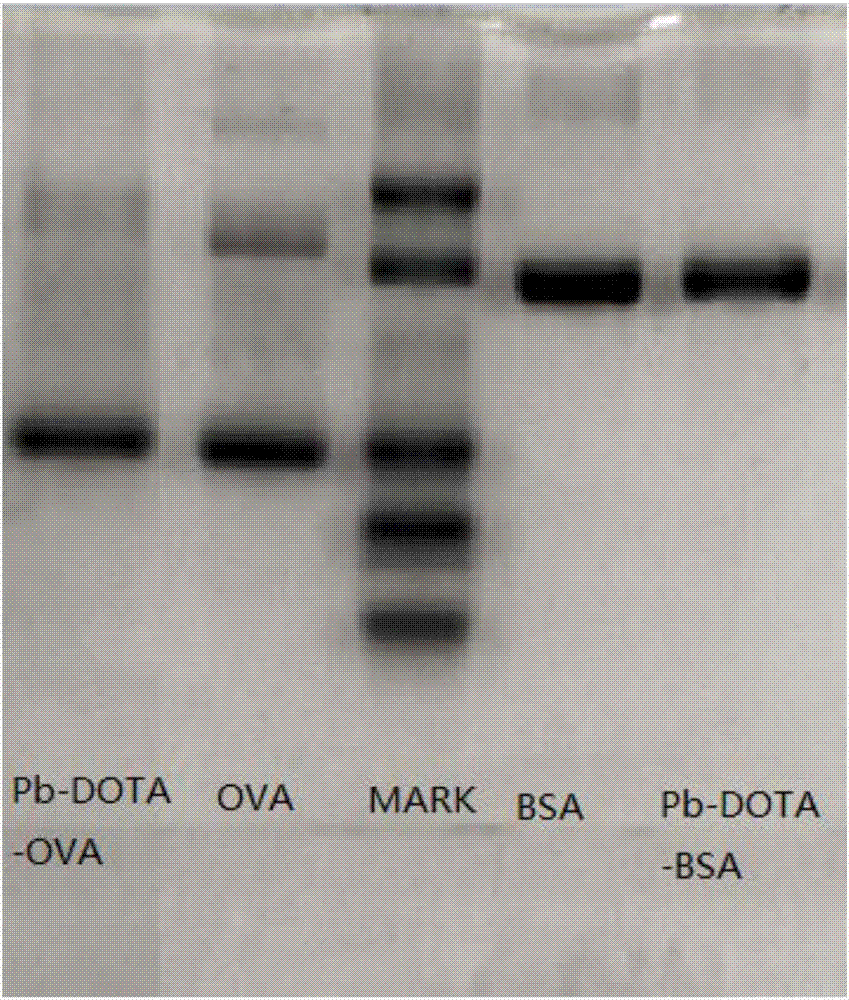
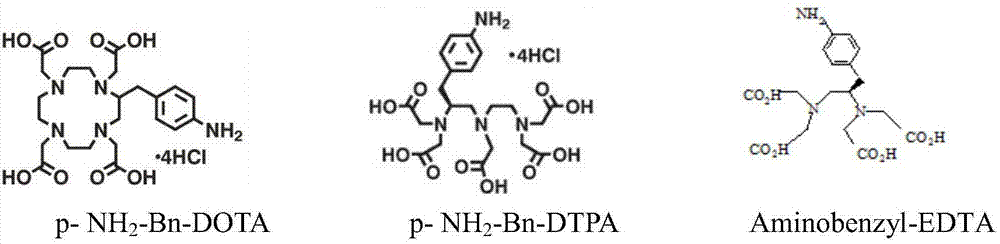
Improved synthesis method of heavy metal lead artificial antigen and application of DOTA (2-S-(4-amino-benzene)-1,4,7,10-tetraazacyclononane-1,4,7,10-tetraacetic acid) to preparation of heavy metal lead artificial antigen reagent
A technology of artificial antigen and synthesis method, which is applied in the direction of peptide preparation method, chemical instrument and method, hybrid peptide, etc., can solve the problem of weak characteristic functional groups, weak antigenic determinant antigenic characteristics, affecting high specificity and high Sensitivity, antibody preparation efficiency and other issues, to achieve high affinity and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 7 mg DOTA, dissolve it in 2 ml 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) solution (0.01 mol / L, pH7.4), and prepare DOTA chelating agent solution, this reaction solution is A solution;
[0028] Weigh 124.19 mg lead nitrate and dissolve it in 5 ml ultrapure water to prepare 7.5X 10-2 The lead nitrate solution of M, this reaction solution is B liquid;
[0029] Pipette 170 μl of solution B and add it dropwise to solution A, and react in the dark for 3 hours at room temperature, and this reaction solution is solution C;
[0030] Add 630 μl, 20 mM glutaraldehyde solution dropwise to solution C, and react overnight at room temperature in the dark, this reaction solution is solution D;
[0031] Weigh 20 mg of bovine serum albumin (BSA) and dissolve it in 3 ml of HEPES, stir magnetically at room temperature and mix well, this reaction solution is E solution;
[0032] Add solution D to solution E dropwise, and react in the dark for 24 hours at room temperature, ...
Embodiment 2
[0036] Weigh 7 mg DOTA, dissolve it in 2 ml 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) solution (0.01 mol / L, pH7.4), and prepare DOTA chelating agent solution, this reaction solution is A solution;
[0037] Weigh 124.19 mg lead nitrate and dissolve it in 5 ml ultrapure water to prepare 7.5X 10-2 The lead nitrate solution of M, this reaction solution is B liquid;
[0038] Pipette 170 μl of solution B and add it dropwise to solution A, and react in the dark for 3 hours at room temperature, and this reaction solution is solution C;
[0039] Add 630 μl, 20 mM glutaraldehyde solution dropwise to solution C, and react overnight at room temperature in the dark, this reaction solution is solution D;
[0040] Weigh 20 mg of chicken ovalbumin OVA and dissolve in 3 ml of HEPES, stir magnetically at room temperature and mix well, this reaction solution is liquid E;
[0041] Add solution D to solution E dropwise, and react in the dark for 24 hours at room temperature, then add 170...
Embodiment 3
[0045] Weigh 7 mg DOTA, dissolve it in 2 ml 4-hydroxyethylpiperazineethanesulfonic acid (HEPES) solution (0.01 mol / L, pH7.4), and prepare DOTA chelating agent solution, this reaction solution is A solution;
[0046] Weigh 124.19 mg lead nitrate and dissolve it in 5 ml ultrapure water to prepare 7.5X 10-2 The lead nitrate solution of M, this reaction solution is B liquid;
[0047] Pipette 170 μl of solution B and add it dropwise to solution A, and react in the dark for 3 hours at room temperature, and this reaction solution is solution C;
[0048] Add 630 μl, 20 mM glutaraldehyde solution dropwise to solution C, and react overnight at room temperature in the dark, this reaction solution is solution D;
[0049] Weigh 20 mg of bovine serum albumin (BSA) and dissolve it in 3 ml of HEPES, stir magnetically at room temperature and mix well, this reaction solution is E solution;
[0050] Add liquid D dropwise to liquid E, and react in the dark for 24 hours at room temperature to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com