Nanocarrier medicine, as well as preparation method and application of nanocarrier medicine
A technology of nano-carriers and drugs, applied in the field of nano-carrier drugs and their preparation, to achieve guaranteed effects, significant therapeutic effects, and improved killing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
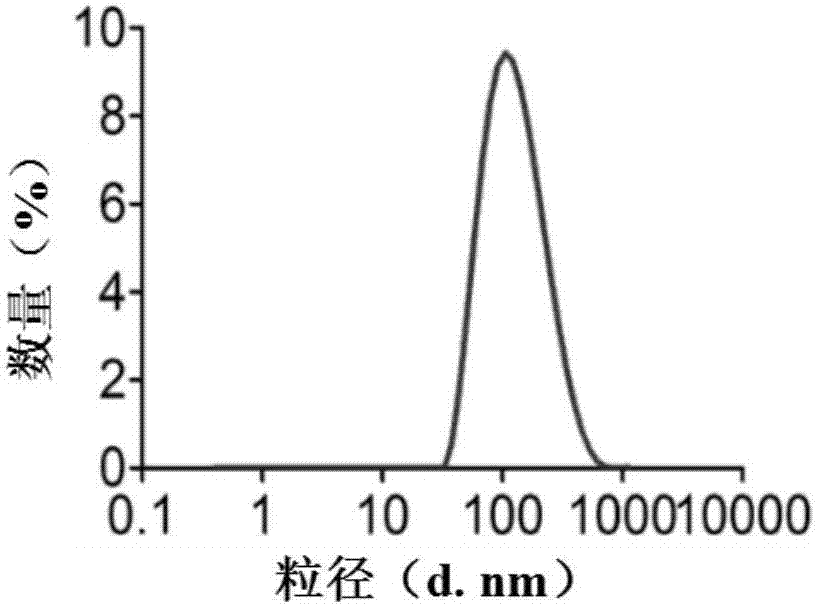
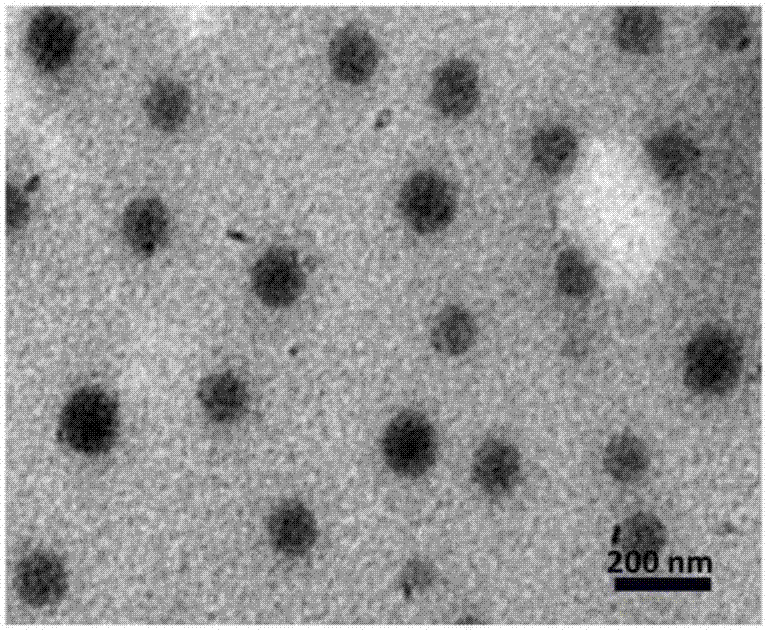
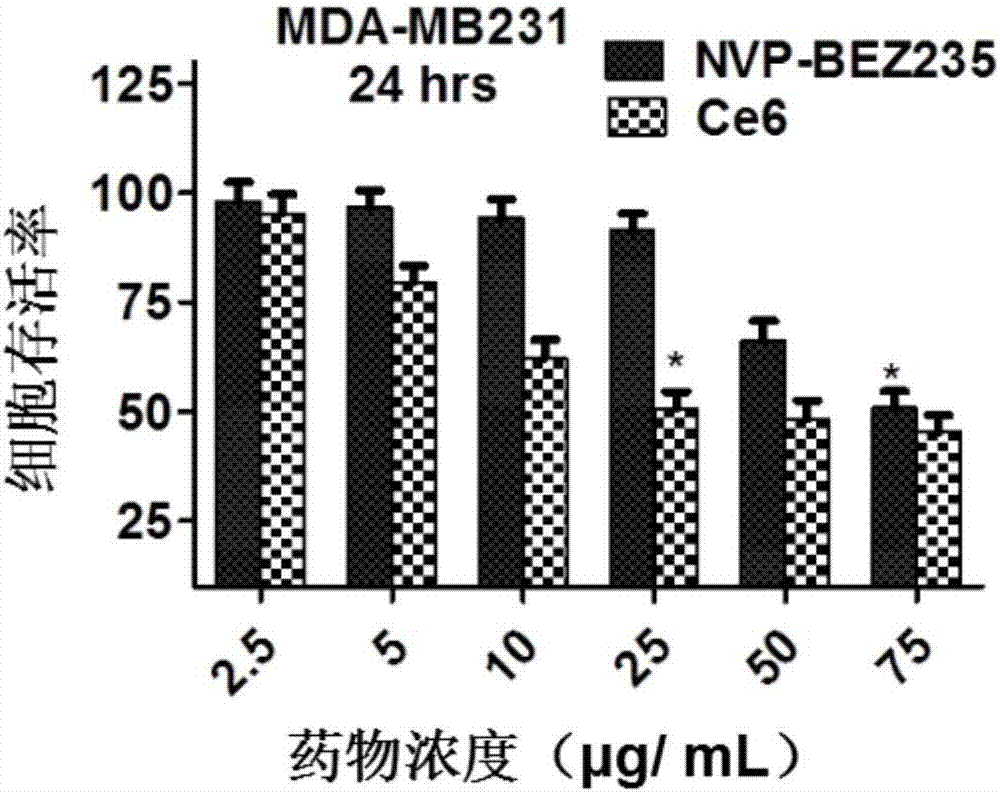
[0056] In this example, the nano-carrier drug uses polylactic acid-glycolic acid (PLGA) as a carrier, loaded with NVP-BEZ235 and chlorin E6 (Ce6), and uses lecithin and distearoylphosphatidylethanolamine - Polyethylene glycol (DSPE-PEG) for PEGylation of the carrier surface.
[0057] The preparation method is as follows:
[0058] (1) Dissolve 0.5mg NVP-BEZ235 and 0.2mg Ce6 in 300μL DMSO to obtain a drug solution;
[0059] (2) 15 mg of polylactic acid-glycolic acid was dissolved in 700 μL of acetonitrile to obtain a carrier solution;
[0060] (3) Mix the drug solution obtained in step (1) with the carrier solution obtained in step (2), and ultrasonicate for 2 minutes to form an oil phase solution;
[0061] (4) 3 mg of lecithin and 3 mg of distearoylphosphatidylethanolamine-polyethylene glycol were dissolved in 5 mL of deionized water to obtain an aqueous phase solution;
[0062] (5) Add the oil phase solution obtained in step (3) to the water phase solution obtained in step ...
Embodiment 2
[0067] In this example, the nano-carrier drug uses polylactic acid-glycolic acid (PLGA) as a carrier, loaded with NVP-BEZ235 and chlorin E6 (Ce6), and uses lecithin and distearoylphosphatidylethanolamine - Polyethylene glycol (DSPE-PEG) for PEGylation of the carrier surface.
[0068] The preparation method is as follows:
[0069] (1) Dissolve 0.75mg NVP-BEZ235 and 0.5mg Ce6 in 300μL DMSO to obtain a drug solution;
[0070] (2) 20 mg of polylactic acid-glycolic acid was dissolved in 700 μL of acetonitrile to obtain a carrier solution;
[0071] (3) Mix the drug solution obtained in step (1) with the carrier solution obtained in step (2), and ultrasonicate for 4 minutes to form an oil phase solution;
[0072] (4) 5 mg of lecithin and 5 mg of distearoylphosphatidylethanolamine-polyethylene glycol were dissolved in 5 mL of deionized water to obtain an aqueous phase solution;
[0073] (5) Add the oil phase solution obtained in step (3) to the water phase solution obtained in step...
Embodiment 3
[0076] In this example, the nano-carrier drug uses polylactic acid-glycolic acid (PLGA) as a carrier, loaded with NVP-BEZ235 and chlorin E6 (Ce6), and uses lecithin and distearoylphosphatidylethanolamine - Polyethylene glycol (DSPE-PEG) for PEGylation of the carrier surface.
[0077] The preparation method is as follows:
[0078] (1) Dissolve 1.2mg NVP-BEZ235 and 0.6mg Ce6 in 300μL DMSO to obtain a drug solution;
[0079] (2) 25 mg of polylactic acid-glycolic acid was dissolved in 700 μL of acetonitrile to obtain a carrier solution;
[0080] (3) Mix the drug solution obtained in step (1) with the carrier solution obtained in step (2), and ultrasonicate for 6 minutes to form an oil phase solution;
[0081] (4) 7.5 mg of lecithin and 7.5 mg of distearoylphosphatidylethanolamine-polyethylene glycol were dissolved in 5 mL of deionized water to obtain an aqueous phase solution;
[0082] (5) Add the oil phase solution obtained in step (3) to the water phase solution obtained in s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com