Preparation method of potassium fluoride
A technology of potassium fluoride and fluorosilicic acid, applied in directions such as alkali metal fluorides, can solve problems such as high production cost, reduce raw material cost and processing cost, and be beneficial to large-scale production and application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In order to solve this problem, the present invention proposes a kind of preparation method of Potassium Fluoride, wherein, the step of this preparation method is as follows:
[0029] (1) First, add a fluorosilicic acid solution with a mass fraction of 20% to 36% in a reaction kettle, start the mixer and stir evenly, then add potassium fluorosilicate solution into the reaction kettle, and continue stirring until the reaction kettle The solution in is homogeneous. Wherein, the equivalent ratio of the added potassium fluorosilicate solution to the fluorosilicate solution ranges from 1:5 to 3:5.
[0030] After adding the above-mentioned potassium fluorosilicate solution, a potassium hydroxide solution with a mass fraction of 48% was added into the reaction kettle at a uniform speed. Wherein, the equivalent ratio of the added potassium hydroxide solution to the added fluosilicic acid solution ranges from 1:1 to 21:1. The main purpose of adding potassium hydroxide solution...
Embodiment 1
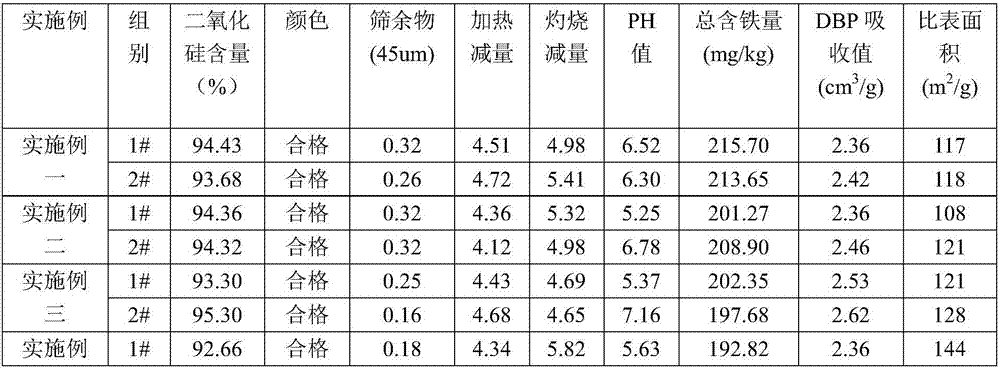
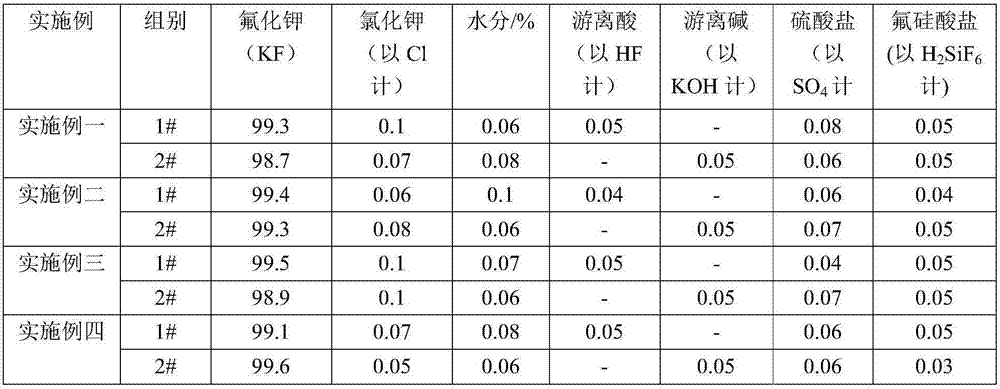
[0050] 1. Add 35% fluorosilicic acid (wherein the fluorosilicic acid is a by-product of the anhydrous hydrogen fluoride production line) into the reactor, and then start the mixer for stirring. After stirring evenly, continue to add potassium fluorosilicate into the reaction kettle, and continue stirring until the mixed solution in the reaction kettle is uniform. In this embodiment, the equivalent ratio between potassium fluorosilicate and fluorosilicic acid is 5:1.
[0051] 2. Add potassium hydroxide solution with a mass fraction of 48% into the above-mentioned reaction kettle at a uniform speed, and continue stirring until the reaction is complete, and the pH value at the end of the reaction is 7.5-8. After the reaction in the reaction kettle is complete, the reaction liquid in the reaction kettle is subjected to solid-liquid separation, wherein the obtained solid is subjected to beating and cleaning treatment to obtain high-purity silica, and the high-purity silica can be o...
Embodiment 2
[0056] 1. Add fluorosilicic acid with a mass fraction of 20% (wherein the fluorosilicic acid is a by-product on the anhydrous hydrogen fluoride production line) into the reaction kettle, and then start the mixer for stirring operation. After stirring evenly, continue to add potassium fluorosilicate with a mass fraction of 48% into the reactor, and continue stirring until the mixed solution in the reactor is uniform. In this embodiment, the equivalent ratio between potassium fluorosilicate and fluorosilicic acid is 3:1.
[0057] 2. Add potassium hydroxide solution with a mass fraction of 48% into the above-mentioned reaction kettle at a uniform speed, and continue to stir until the reaction is complete, and the pH value at the end of the reaction is 8 to 8.5. After the reaction in the reaction kettle is complete, the reaction liquid in the reaction kettle is subjected to solid-liquid separation, wherein the obtained solid is subjected to beating and cleaning treatment to obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com