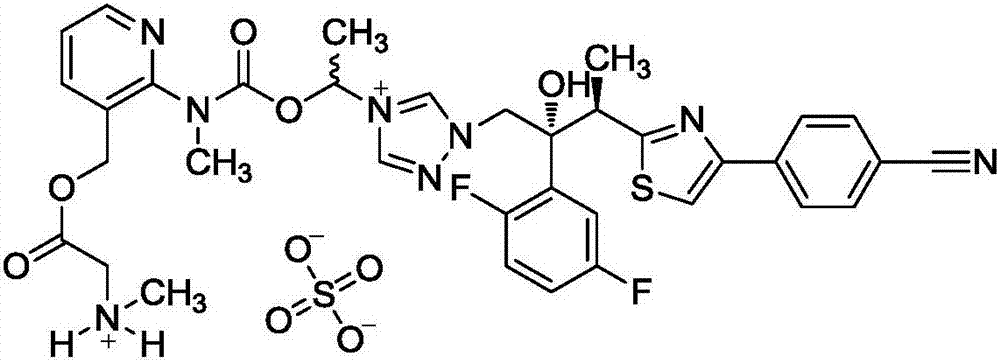
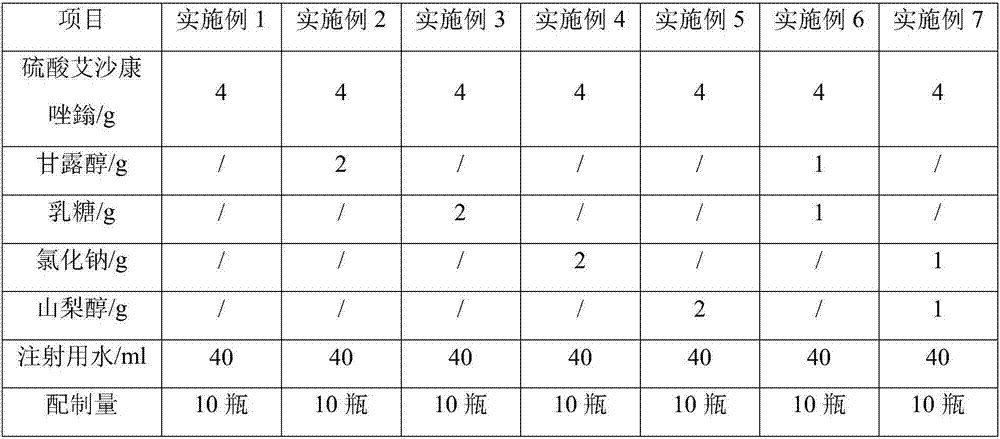
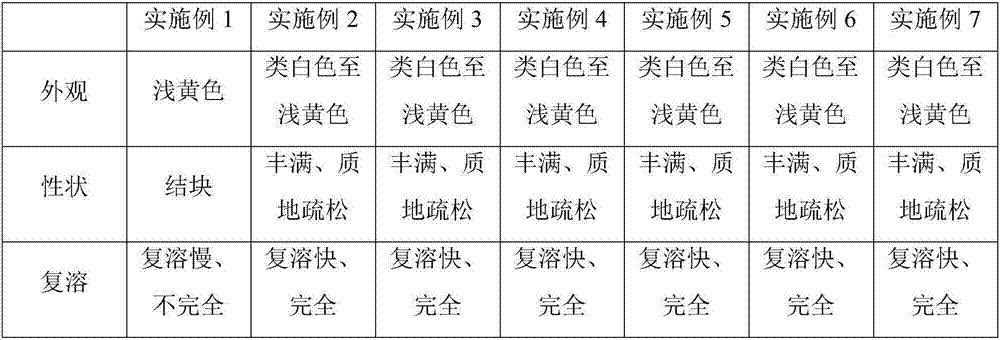
Freeze-dried isavuconazonium sulfate powder injection and preparation method
A technology of isavuconazolium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of nephrotoxicity risk, cumbersome preparation process, and complex composition, and achieve plump appearance, improved curative effect, and convenient transportation and storage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8~9
[0045] Table 3. Embodiment 8~9 prescription
[0046] project
Example 8
Example 9
Example 10
Isavuconazolium sulfate / g
4
4
4
Mannitol / g
0.8
1
1
concentrated sulfuric acid
/
/
/
Water for injection / ml
30
40
30
Preparation amount
10 bottles
10 bottles
10 bottles
[0047] Preparation method: preparation method: take 50-90% of the water for injection to dissolve the excipient (one or more in mannitol, lactose, sodium chloride, sorbitol), and after all dissolve, reduce the temperature to 0-15 ℃, add isavuconazolium sulfate, stir to dissolve into a clear solution, adjust the pH to 1.2-1.8 with sulfuric acid, add water for injection to constant volume, then sterile filter and pack. After half-press stoppering, cool to -40°C, keep warm for 3 hours, heat up to -15°C~0°C for sublimation and drying for 5 hours, then raise the temperature to 20~40°C, analyze and dry for 8~20 hours, t...
Embodiment 11
[0050] Example 11 Preparation of isavuconazolium sulfate freeze-dried powder for injection, 100 bottles in total
[0051] prescription:
Embodiment 11
[0052] Table 5. Embodiment 11 prescription
[0053] project
Example 11
Isavuconazolium sulfate / g
40
Mannitol / g
10
concentrated sulfuric acid
/
Water for injection / ml
300
Preparation amount
100 bottles
[0054]Preparation method: preparation method: take 50-90% of the water for injection to dissolve the excipient (one or more in mannitol, lactose, sodium chloride, sorbitol), and after all the dissolution, reduce the temperature to 0-15 ℃, add isavuconazolium sulfate, stir to dissolve into a clear solution, adjust the pH to 1.5 with sulfuric acid, add water for injection to make up the volume, then sterile filter and pack. After half-press stoppering, cool to -40°C, keep warm for 3 hours, heat up to -15°C~0°C for sublimation and drying for 5 hours, then heat up to 20~40°C, analyze and dry for 8~20 hours, then cool down to 5-10°C, keep warm After 1 hour, stoppering and coming out of the cabinet, the freeze-dryi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com