Synthesis method of 2-bromo-3-fluobenzoic acid
The technology of a kind of fluorobenzoic acid and synthetic method is applied in the field of synthesis of 2-bromo-3-fluorobenzoic acid, which can solve the problems of increased production cost, unfriendly environment, cumbersome steps, etc., and achieve low cost, cheap raw materials, and Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
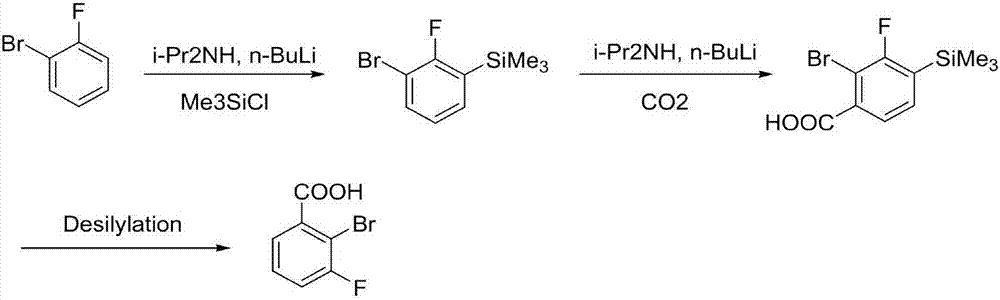
[0029] In the first step, add 160mL tetrahydrofuran and diisopropylamine (24.3g, 0.24mol) to the reaction flask, cool down to -25°C, and add n-butyllithium (96mL, 0.24mol, 2.5mol / L) dropwise under nitrogen protection , the temperature is controlled not to exceed -10°C, after the drop is completed, the solution is kept and stirred for 30 minutes to obtain a tetrahydrofuran solution of lithium diisopropylamide. The reaction solution was cooled to -78°C, controlled at -78 to -65°C, o-bromofluorobenzene (35.0 g, 0.2 mol) was added dropwise, kept stirring for 1 hour, and trimethylchlorosilane ( 32.6g, 0.3mol), temperature control -78~-65°C, after dropping, stir at room temperature for 1 hour, dropwise add 1N HCl solution to quench the reaction, control temperature + , used directly in the next step without purification.
[0030] In the second step, 200 mL of tetrahydrofuran and diisopropylamine (21.1 g, 0.209 mol) were added to the reaction flask, the temperature was lowered to -20...
Embodiment 2
[0033] In the first step, 160 mL of cyclopentyl methyl ether and diisopropylamine (22.3 g, 0.22 mol) were added to the reaction flask, the temperature was lowered to -40 ° C, and n-butyllithium (88 mL, 0.22 mol, 2.5mol / L), the temperature was controlled not to exceed -10°C, after the drop was completed, the solution was kept and stirred for 30 minutes to obtain a cyclopentyl methyl ether solution of lithium diisopropylamide. The reaction solution was cooled to -78°C, controlled at -78 to -40°C, o-bromofluorobenzene (35.0 g, 0.2 mol) was added dropwise, kept stirring for 1 hour, and trimethylchlorosilane ( 43.5g, 0.4mol), temperature control -78~-40°C, after dropping, stir at room temperature for 1 hour, dropwise add 1N HCl solution to quench the reaction, temperature control <20°C, add 160mL n-heptane for layering, organic phase subtraction The solvent was concentrated under reduced pressure to obtain 46.6 g of yellow liquid 3-bromo-2-fluorophenyltrimethylsilane with a purity ...
Embodiment 3
[0037] In the first step, add 160mL 2-methyltetrahydrofuran and diisopropylamine (22.3g, 0.22mol) to the reaction flask, cool down to -40°C, and add n-butyl lithium (88mL, 0.22mol, 2.5 mol / L), the temperature was controlled not to exceed -10°C, after the drop was completed, the solution was kept and stirred for 30 minutes to obtain a solution of lithium diisopropylamide in 2-methyltetrahydrofuran. The reaction solution was cooled to -78°C, controlled at -78 to -40°C, o-bromofluorobenzene (35.0 g, 0.2 mol) was added dropwise, kept stirring for 1 hour, and trimethylchlorosilane ( 65.2g, 0.6mol), temperature control -78~-40°C, after dropping, stir at room temperature for 1 hour, dropwise add 1N HCl solution to quench the reaction, control temperature <20°C, add 160mL n-heptane for layering, organic phase subtraction The solvent was concentrated under reduced pressure to obtain 46.7 g of yellow liquid 3-bromo-2-fluorophenyltrimethylsilane with a purity of 94.1%, which was directly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com