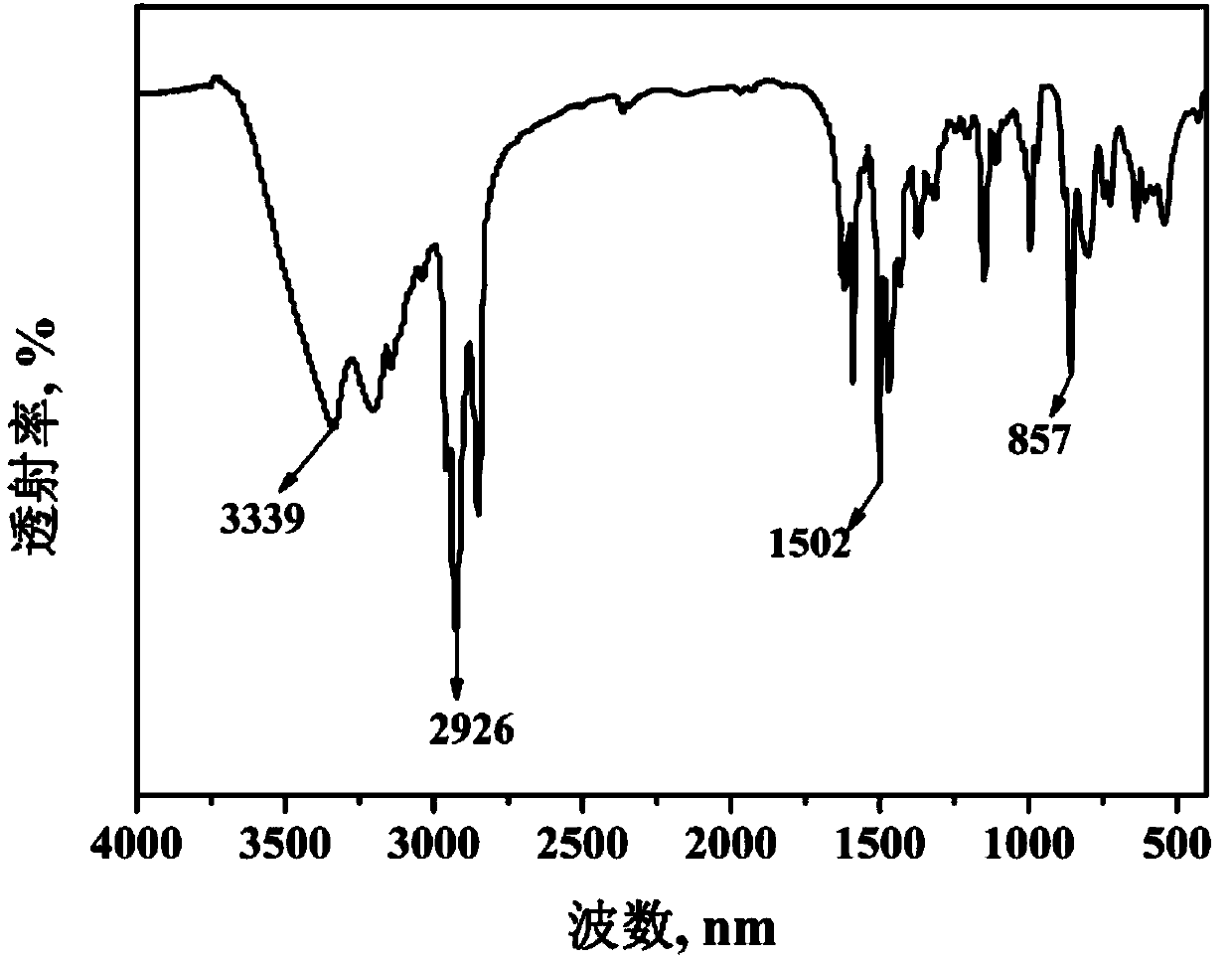
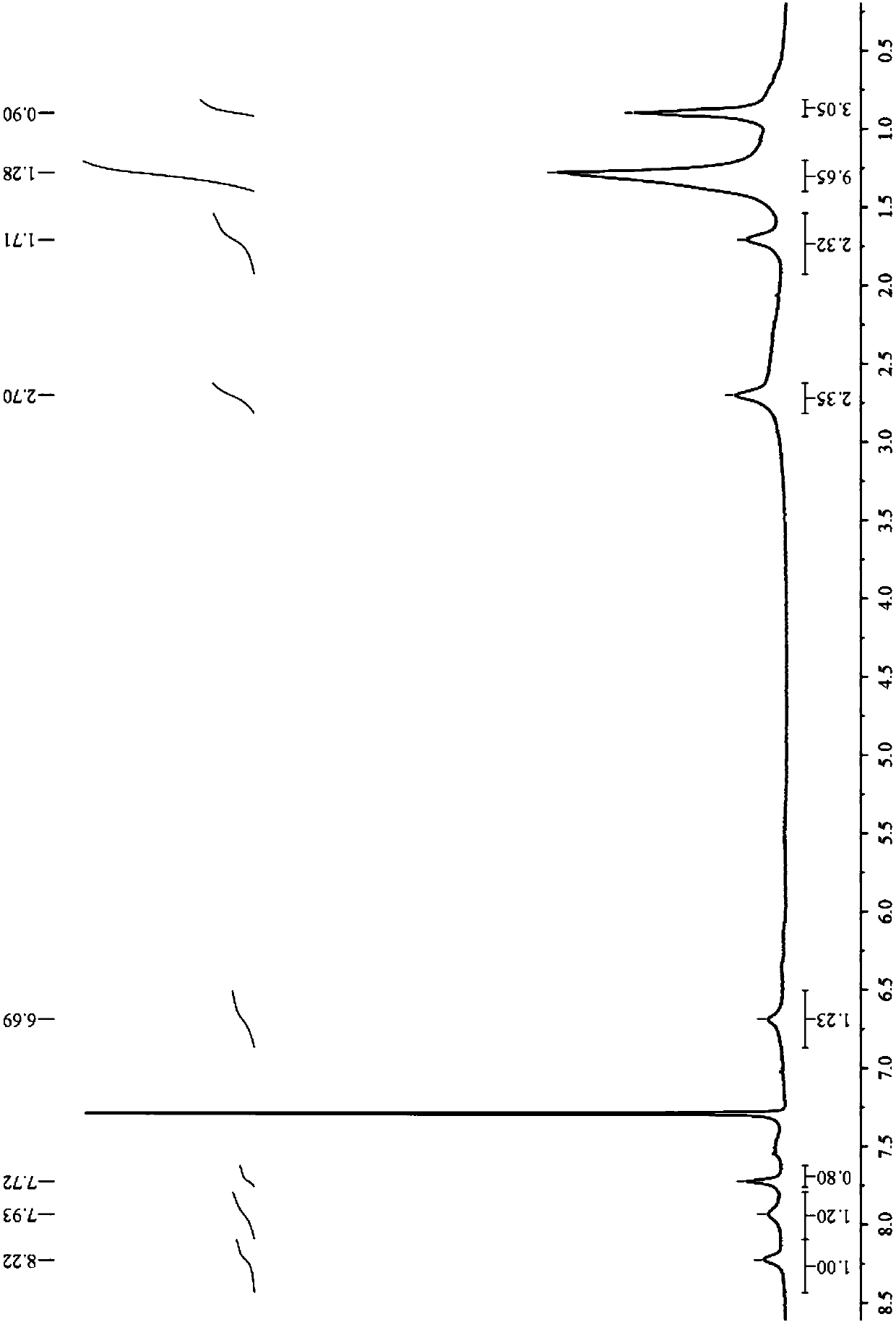
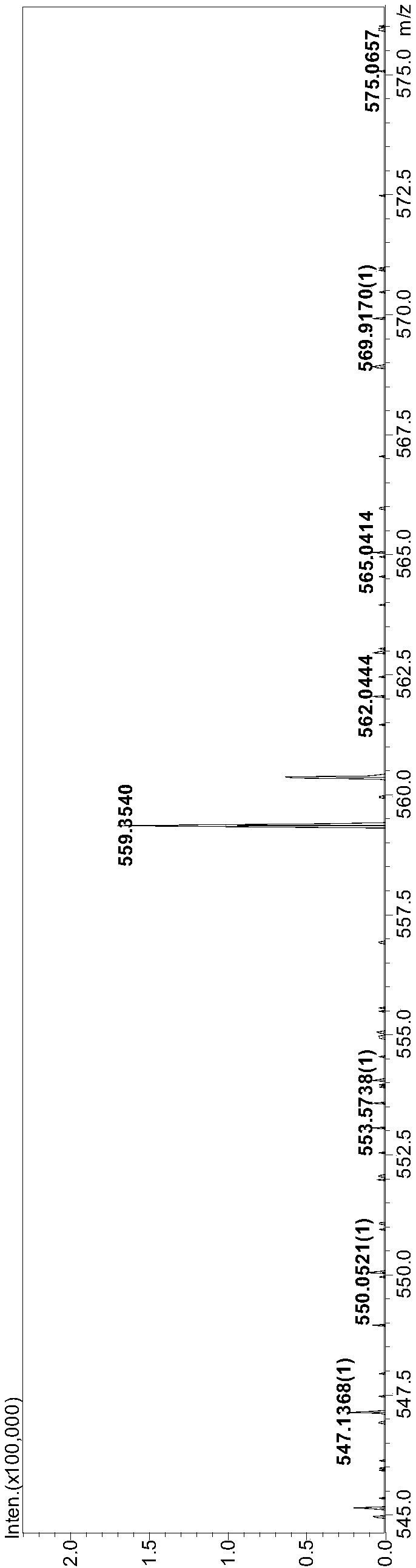
Double-pyrazole extracting agent derived from o-phenanthroline, and preparation method and application of double-pyrazole extracting agent
A technology of o-phenanthroline and extraction agent, which is applied in the fields of nuclear fuel cycle and nuclear waste treatment, and can solve the problems of difficult stripping and slow extraction kinetics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] This example prepares 2,9-bis(5-n-octyl-1-hydrogen-pyrazole)-1,10-phenanthroline (nOct-BPPhen) with structural formula (13), and its synthetic route is as follows Shown:
[0049]
[0050] The preparation process of above-mentioned nOct-BPPhen comprises the following steps:
[0051] (1) Under stirring conditions, add selenium dioxide (5.3g, 48mmol) into a complex solvent composed of 150mL 1,4-dioxane and 10mL water, heat up to reflux, and then dropwise add 2,9- Dimethyl-1,10-phenanthroline in 1,4-dioxane solution [from 2,9-dimethyl-1,10-phenanthroline (5.0g, 24mmol) dissolved in 100mL 1,4-dioxane to obtain], after the dropwise addition, react for 3.0h, then filter while hot, place the obtained filtrate to cool until solid precipitates, and then filter the filtrate to obtain a reddish-brown flocculent solid, namely 2, 9-dicarbaldehyde-1,10-phenanthroline (4.8g, yield 85%);
[0052] (2) Under stirring conditions, add 4.8 g of 2,9-dicarbaldehyde-1,10-phenanthroline ob...
Embodiment 2
[0062] This example prepares 2,9-bis(5-n-butyl-1-hydrogen-pyrazole)-1,10-phenanthroline (nBu-BPPhen) with structural formula (14), and its synthetic route is as follows Shown:
[0063]
[0064] The preparation process of above-mentioned nBu-BPPhen comprises the following steps:
[0065] (1) Under stirring conditions, KMnO 4 (7.6g, 48mmol) was added to 120mL of dichloromethane, heated to reflux, and then a dichloromethane solution of 2,9-dimethyl-1,10-phenanthroline was added dropwise [from 2,9-di Methyl-1,10-phenanthroline (5.0g, 24mmol) was dissolved in 100mL of dichloromethane to obtain], after the dropwise addition was completed, reacted for 4.0h, then filtered while it was hot, and the obtained filtrate was left to cool until solids were precipitated. Then the filtrate was filtered to obtain a reddish-brown flocculent solid, namely 2,9-dicarbaldehyde-1,10-phenanthroline (3.5 g, yield 62%);
[0066] (2) Under stirring conditions, add 3.5 g of 2,9-dicarbaldehyde-1,10-p...
Embodiment 3
[0071] This embodiment prepares 2,9-bis(5-isobutyl-1-hydrogen-pyrazole)-1,10-phenanthroline (iBu-BPPhen) with structural formula (15), and its synthetic route is as follows Shown:
[0072]
[0073] The preparation process of above-mentioned iBu-BPPhen comprises the following steps:
[0074] (1) Under stirring conditions, K 2 Cr 2 o 7 (14g, 48mmol) was added to 150mL tetrahydrofuran, heated to reflux, and then dropwise added a solution of 2,9-dimethyl-1,10-phenanthroline in tetrahydrofuran [from 2,9-dimethyl-1, 10-Phenanthroline (5.0g, 24mmol) was dissolved in 100mL tetrahydrofuran to obtain], after the dropwise addition was completed, reacted for 5.0h, then filtered while it was hot, and the obtained filtrate was left to cool until solids were precipitated, and then filtered the filtrate to obtain red Brown flocculent solid, namely 2,9-dicarbaldehyde-1,10-phenanthroline (4.5 g, yield 80%);
[0075] (2) Under stirring conditions, add 4.5 g of 2,9-dicarbaldehyde-1,10-phena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com