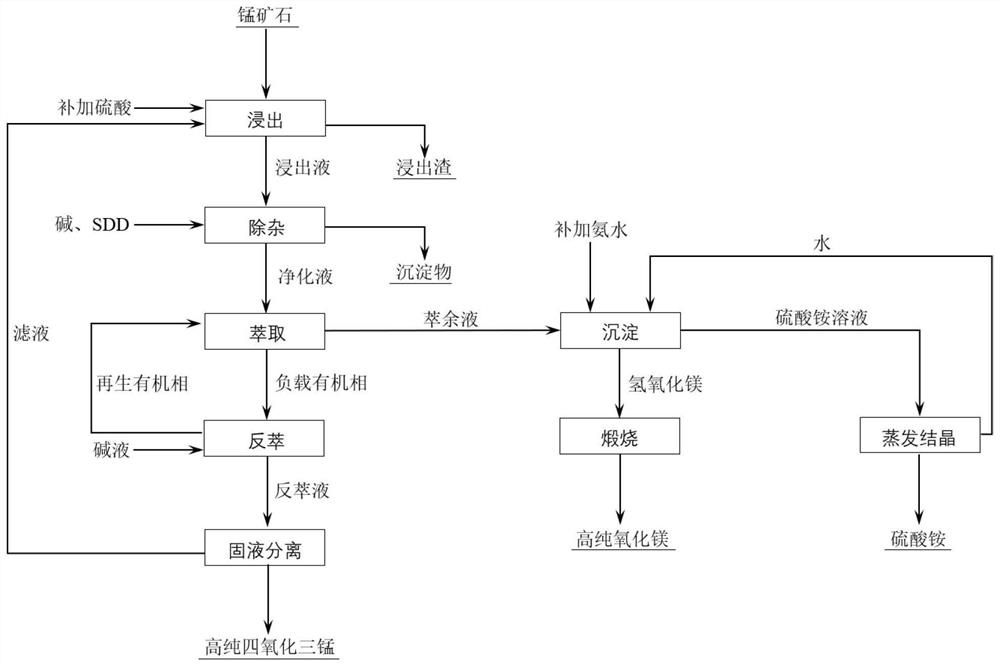
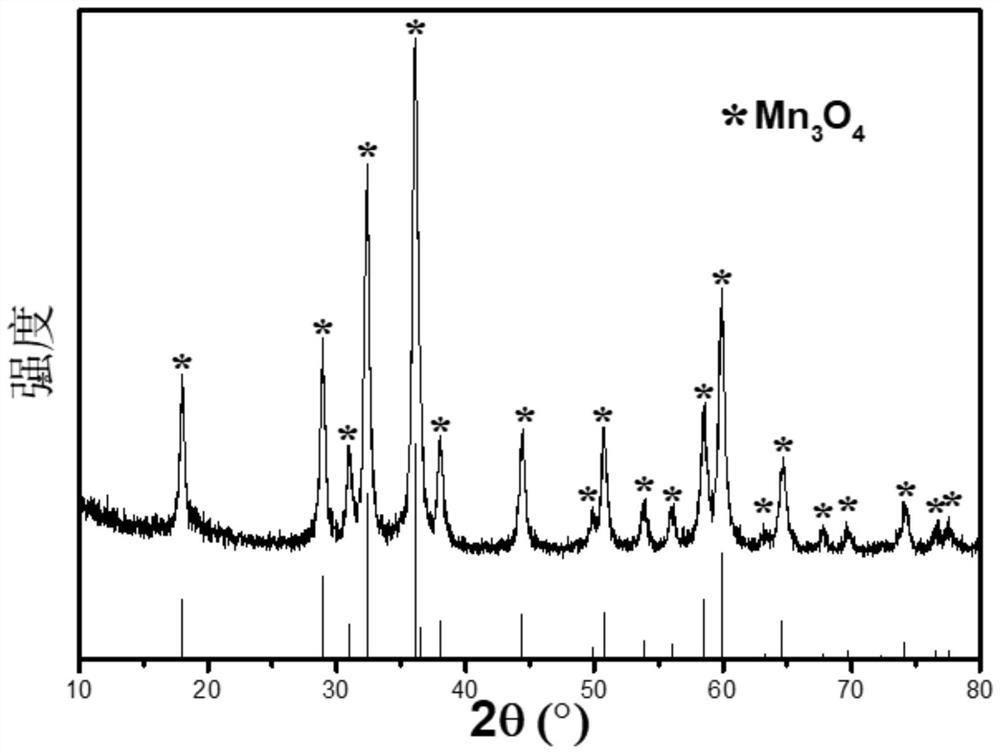
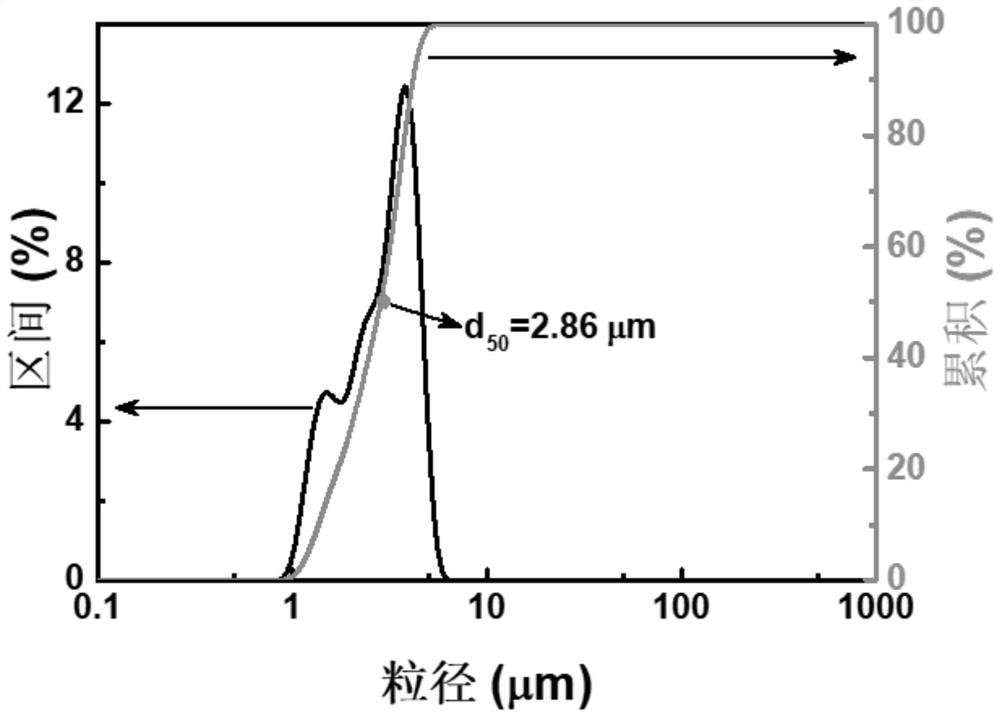
Method for preparing high-purity manganous-manganic oxide and high-purity magnesium oxide
A technology of manganese tetroxide and magnesium oxide, applied in the directions of manganese oxide/manganese hydroxide, magnesium oxide, ammonium sulfate, etc., can solve the problems of waste of magnesium resources, poor quality stability, high energy consumption, and save reagent consumption. and energy consumption, reducing operation steps, and the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kind of production method of electrolytic manganese
[0054] Including the processes of leaching, impurity removal, extraction, stripping and recovery of ammonia nitrogen and magnesium, the details are as follows:
[0055] S1. Leaching: Dilute 18.23g of concentrated sulfuric acid to 95mL with water, then add 30g of crushed manganese ore powder, stir and leaching at 90°C for 3 hours, and filter to obtain the leaching solution and leaching residue. The rate is 96.34%. In the leachate, the concentration of each element is: Mn 35.44g / L, Mg 9.14g / L, Fe 2.96g / L, Na 0.38g / L, Ca0.33g / L, Al 0.11g / L;
[0056] S2, impurity removal: add manganese dioxide to oxidize low-valent iron in the leaching solution obtained in step S1, the ratio of the amount of substance added by manganese dioxide to the amount of low-valent iron in the leaching solution is 0.5:1, then add sodium hydroxide Adjust the pH value to 5.47, precipitate Fe and Al ions, and then add SDD to react to form chelated...
Embodiment 2
[0063] A kind of production method of electrolytic manganese
[0064] Including the processes of leaching, impurity removal, extraction, stripping and recovery of ammonia nitrogen and magnesium, the details are as follows:
[0065] S1. Leaching: Dilute 18.19g of concentrated sulfuric acid to 130mL with water, then add 30g of crushed manganese ore powder, stir and leaching at 50°C for 5h, and filter to obtain the leaching solution and leaching residue. The rate is 97.41%. In the leach solution, the concentration of each element is: Mn 26.19g / L, Mg 6.83g / L, Fe 2.19g / L, Na 0.28g / L, Ca0.24g / L, Al 0.08g / L;
[0066] S2, impurity removal: add hydrogen peroxide to the leaching solution obtained in step S1 to oxidize the low-valent iron, the ratio of the amount of the substance added to the hydrogen peroxide to the amount of the low-valent iron in the leaching solution is 0.5:1, then add sodium hydroxide to adjust the pH value to 5.26. Precipitate Fe and Al ions, and then add SDD to r...
example 3
[0073] The production method of the electrolytic manganese of the present embodiment is the same as that of Example 1, the difference is that in step S3, neodecanoic acid is used as the extraction agent, aviation kerosene is the diluent, the concentration is 400g / L, and the mass fraction is 30%. Sodium hydroxide solution is saponified to a saponification rate of 20%, the pH of the purified solution is adjusted to be 4, and O:A=1:1 is controlled in the countercurrent extraction process, and the purified solution obtained in step S2 and the organic phase with a saponification rate of 20% are subjected to secondary The loaded organic phase and raffinate were obtained by countercurrent extraction. The manganese concentration in the organic phase was detected to be 8.79g / L, and the magnesium concentration was 0.73g / L. The loaded organic phase was washed with manganese sulfate solution at pH 5.0-5.5 to remove Impurity magnesium in the organic phase is loaded to obtain an organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com