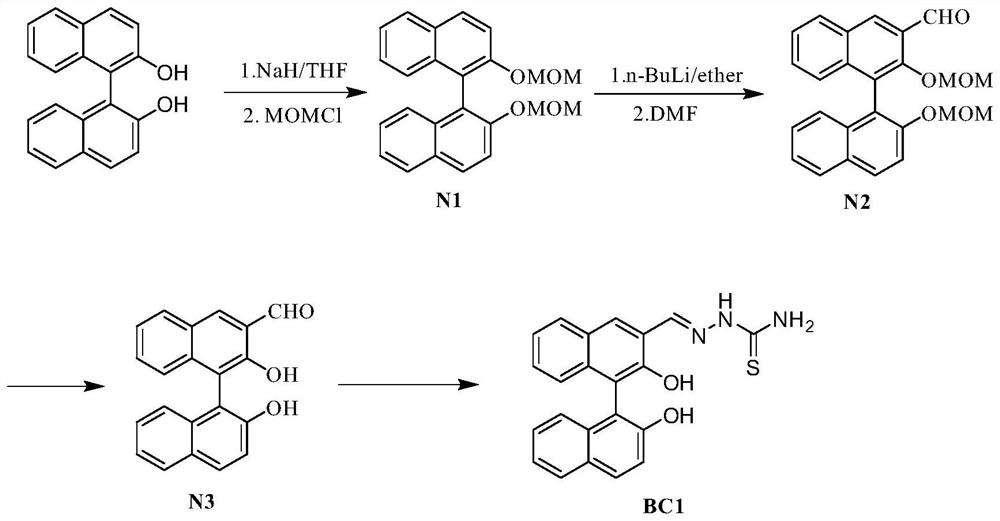
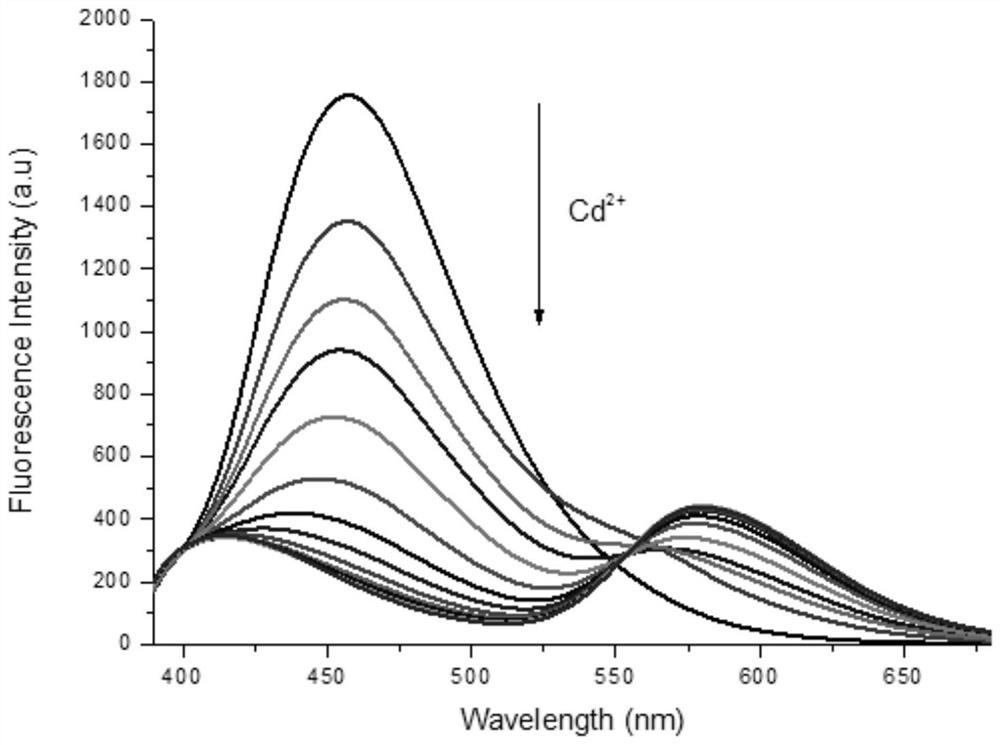
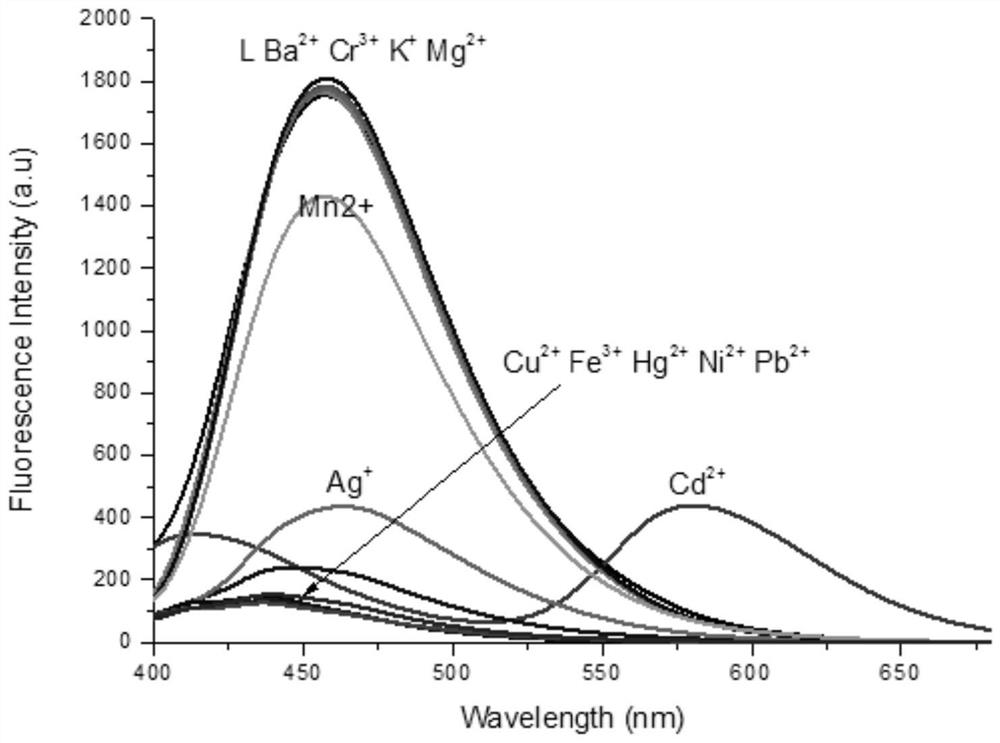
Fluorescent probe based on BINOL, synthetic method of fluorescent probe and application of fluorescent probe in detection of cadmium ions
A technology for fluorescent probes and synthesis methods, applied in the field of fluorescent probes, can solve the problems of poor selectivity and sensitivity of fluorescent probes, complex molecular structure, unstable properties, etc., and achieve improved selectivity and sensitivity and high yield , fast response and recognition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This example provides a BINOL-based fluorescent probe whose chemical name is (E)-2-((2,2'-dihydroxy-[1,1'-binaphthyl]-3-yl)methylene base) hydrazine methyl thioamide, its chemical structural formula is:
[0034]
[0035] This embodiment also provides a method for synthesizing the above-mentioned BINOL-based fluorescent probe. The synthetic route is as follows: figure 1 shown, the specific steps are as follows:
[0036] Synthesis of compound N1: under argon protection, add 20-80 mL of anhydrous THF to a 100-mL three-necked flask, then add 0.5-1.0 g of NaH, stir in an ice bath at 0°C, and then add to the three-necked flask 1~5g binaphthyl-2,2'-diphenol, continue to stir for 10-30min under ice bath at 0℃, slowly add 1~5mL MOMCl to the three-necked flask through a syringe, and finish the dropwise addition for half an hour, remove the ice bath and let it cool down overnight. After the reaction, water was added to the reaction solution to quench the reaction, and ethyl ...
Embodiment 2
[0042] This embodiment provides a method for synthesizing a BINOL-based fluorescent probe BC1. The synthetic route is as follows: figure 1 shown, the specific steps are as follows:
[0043] Synthesis of compound N1: under argon protection, add 40 mL of anhydrously treated THF to a 100 mL three-necked flask, then add 0.76 g (31.5 mmol) of NaH, stir under an ice bath at 0°C, and then add to the three-necked flask Add 3.75g (13.1mmol) of binaphthyl-2,2'-diphenol, continue to stir for 20min under ice bath at 0°C, slowly add 2.4mL of MOMCl into the three-necked flask through a syringe, dropwise for half an hour, remove the ice bath temperature next night. The treatment steps after the reaction are the same as those in Example 1, and 2.48 g of colorless solid 2,2'-methoxymethoxy-1,1'-binaphthyl, that is, compound N1, can be obtained through silica gel chromatographic column separation.
[0044] Synthesis of compound N2: under the protection of nitrogen or argon, dissolve 1.24g (3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com