Artificial three-dimensional epidermis model, and construction method and construction medium thereof
A culture medium and three-dimensional technology, applied in artificial cell constructs, tissue culture, cell culture active agents, etc., can solve the problems of skin irritation test verification, insufficient attention and lag in research on alternative methods, and achieve obvious effects of stratum corneum differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103]
[0104] Human epidermal keratinocytes prepared as above were used as seed cells, digested and separated as above, and then proliferated and differentiated by the above-mentioned gas-liquid phase culture.
[0105] Wherein, the differentiation medium used is containing 10% Fetal Clone II serum, 10ng / ml EGF1, 10 -10 M cholera toxin, 1 μg / ml hydrocortisone, 5 μg / ml bovine insulin, 10 -10 M triiodothyronine, and 5 μg / ml gentamicin in DMEM / F12 medium.
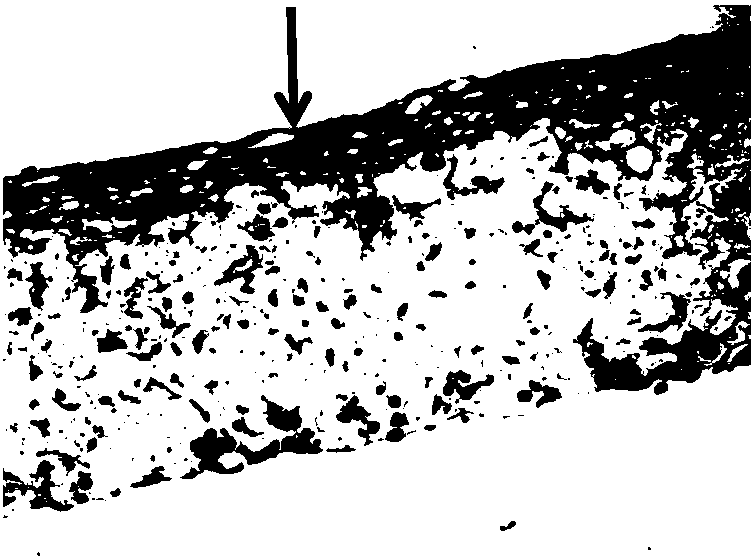
[0106] After being replaced with differentiation medium, the culture was continued for 30 days, and then taken out for paraffin section staining. The slice dyeing figure of the artificial three-dimensional epidermis model of embodiment 1 is as follows figure 1 shown.
[0107] figure 1 The stratum corneum constructed by normal human primary keratinocytes is very obvious and multi-layer differentiated, and the thickness of the stratum corneum is obviously enhanced after 30 days of air-liquid phase culture. Many experimen...
Embodiment 2
[0108]
[0109] Human epidermal keratinocytes prepared as above were used as seed cells, digested and separated as above, and then proliferated and differentiated by the above-mentioned gas-liquid phase culture.
[0110] Wherein, the differentiation medium used is containing 12% Fetal Clone II serum, 12ng / ml EGF1, 10 -11 M cholera toxin, 1.2 μg / ml hydrocortisone, 4 μg / ml bovine insulin, 10 -10 M triiodothyronine, and 5 μg / ml gentamicin in DMEM / F12 medium.
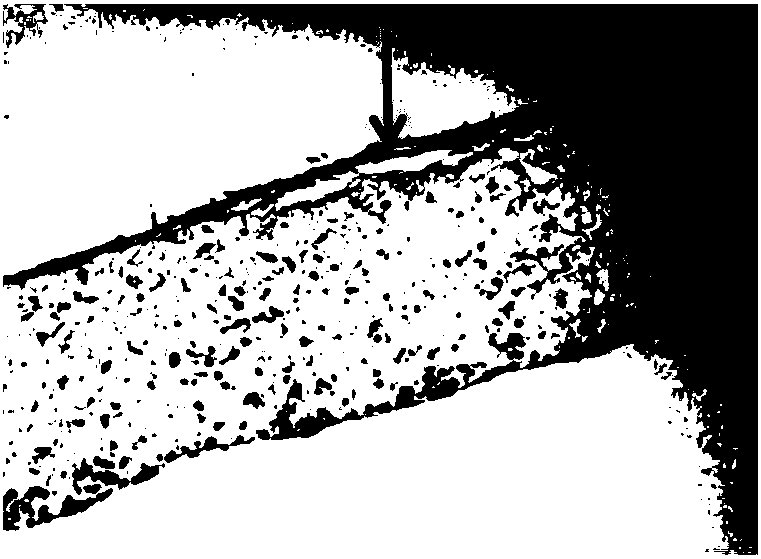
[0111] After being replaced with differentiation medium, the culture was continued for 30 days, and then taken out for paraffin section staining. figure 2 The stratum corneum constructed by normal human primary keratinocytes is relatively obvious, and the thickness of the stratum corneum is obviously enhanced after 30 days of gas-liquid phase culture. figure 2 The black arrows in indicate the thickness of the constructed epidermis.
Embodiment 3
[0112]
[0113] Human epidermal keratinocytes prepared as above were used as seed cells, digested and separated as above, and then proliferated and differentiated by the above-mentioned gas-liquid phase culture.
[0114] Wherein, the differentiation medium used is containing 8% Fetal Clone II serum, 8ng / ml EGF1, 10 -9 M cholera toxin, 0.8 μg / ml hydrocortisone, 6 μg / ml bovine insulin, 10 -10 M triiodothyronine, and DMEM / F12 medium of 5 μg / ml gentamicin.
[0115] After being replaced with differentiation medium, the culture was continued for 30 days, and then taken out for paraffin section staining. The section dyeing figure and the artificial three-dimensional epidermis model of embodiment 1 figure 1 similar.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com