L-lactate dehydrogenase mutant with improved catalysis efficiency and building method thereof
A technology for improving the catalytic activity of lactate dehydrogenase, which is applied in the field of genetic engineering and can solve the problems of limiting the wide application of L/D-LDH and low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Construction of mutant enzyme gene and its expression plasmid
[0025] The amplified nucleotide sequence is the gene Lcldh shown in SEQ ID NO.4, the amplified product is ligated with pUCm-T, transformed into E.coli JM109, and subjected to blue-white screening, PCR verification of bacteria solution and DNA sequencing. The recombinant plasmid with correct sequencing was named pUCm-T-Lcldh. Digested pUCm-T-Lcldh with NdeI and XhoI, recovered Lcldh, ligated with pET-28a(+) which had been digested with the same double enzymes, and obtained recombinant plasmid pET-28a(+)-Lcldh.
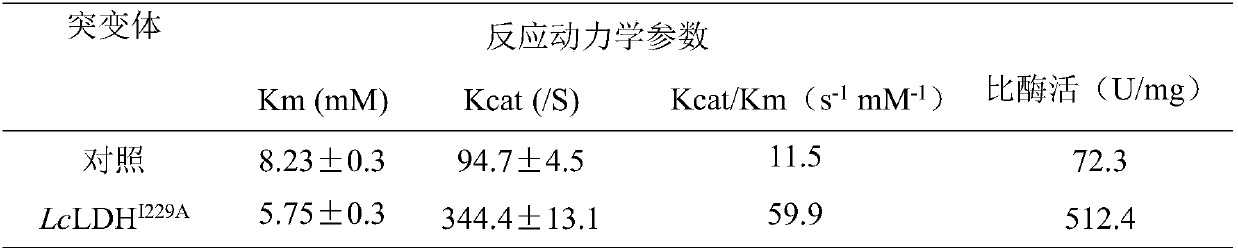
[0026] Using the pET-28a(+)-Lcldh recombinant plasmid as a template, using F1primer (sequence shown in SEQ ID NO.5) and R1primer (sequence shown in SEQ ID NO.6) as primers, site-directed mutagenesis was performed by PCR to obtain A recombinant plasmid carrying the mutant gene, named pET-22b(+)-Lcldh I229A .
Embodiment 2
[0027] Embodiment 2: L-lactate dehydrogenase-producing Escherichia coli engineering bacteria construction
[0028] The recombinant plasmid pET-22b(+)-Lcldh that embodiment 1 obtains I229A To transform E.coli BL21 competent cells, the specific method is as follows:
[0029] 1) Inoculate activated E.coli BL21 on LB plate in 2 mL LB medium, culture overnight at 37°C, 220r / min; inoculate 2% of the above culture solution in 5mL LB medium, culture at 37°C, 220r / min for 4h;
[0030] 2) Take 1.4mL of the above bacterial solution and put it into a 1.5mLEP tube, bathe in ice for 10min, centrifuge at 4000r / min for 2min, and collect the bacteria;
[0031] 3) Add 1mL of pre-cooled 0.1M CaCl 2 The above-mentioned cells were resuspended in the solution, placed in an ice bath for 10 minutes, centrifuged at 4000 r / min for 2 minutes, and the bacteria were collected;
[0032] 4) Add 100 μL of pre-cooled 0.1M CaCl 2 Suspend the above cells in the solution and store at 4°C for 30 minutes to tr...
Embodiment 3
[0038] Example 3: Recombinant Escherichia coli induced expression
[0039] Activate the recombinant bacteria LcLdhBL21 constructed in Example 2 on the LB plate, pick a single colony into 2mL LB medium, culture at 37°C and 220r / min for 12h, and inoculate 100mL / 500mL LB medium according to 2% inoculum size, After culturing at 220r / min for 2 hours at 37°C, add IPTG to a final concentration of 0.4mM, induce expression at 20°C for 8h, centrifuge at 8000r / min for 5min to collect bacteria, and obtain crude enzyme solution after ultrasonic crushing, and purify the crude enzyme solution with a nickel column. Methods as below:
[0040] 1) Wash the column with ultrapure water for more than 3 times;
[0041] 2) Wash the column 3 times with the newly prepared binding solution;
[0042]3) Sample loading: filter the fermentation broth with a water membrane. If the amount is large, it can be repeatedly passed through the column; the sample volume is <= 8mL, generally about 4mL; the lower le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com