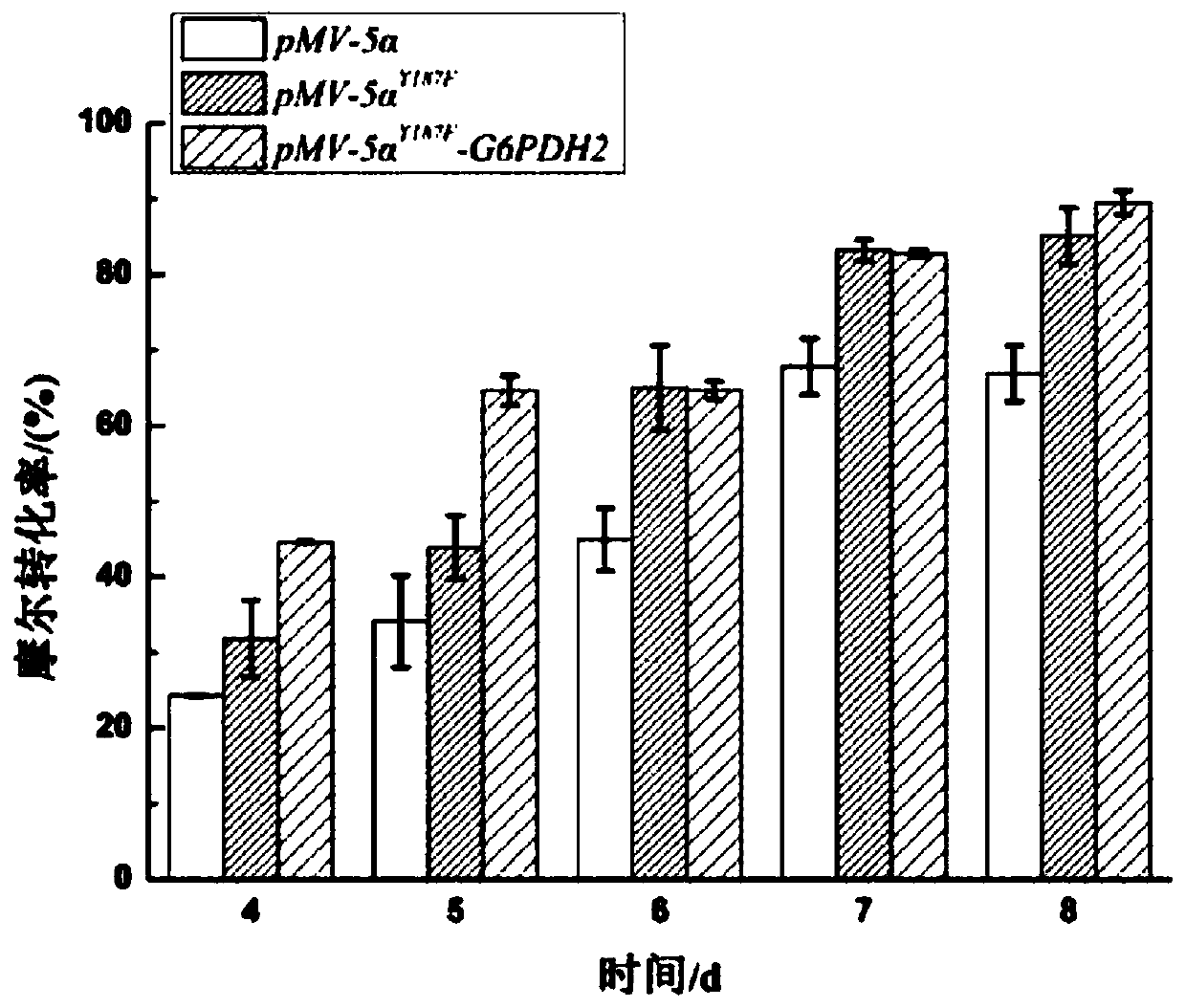
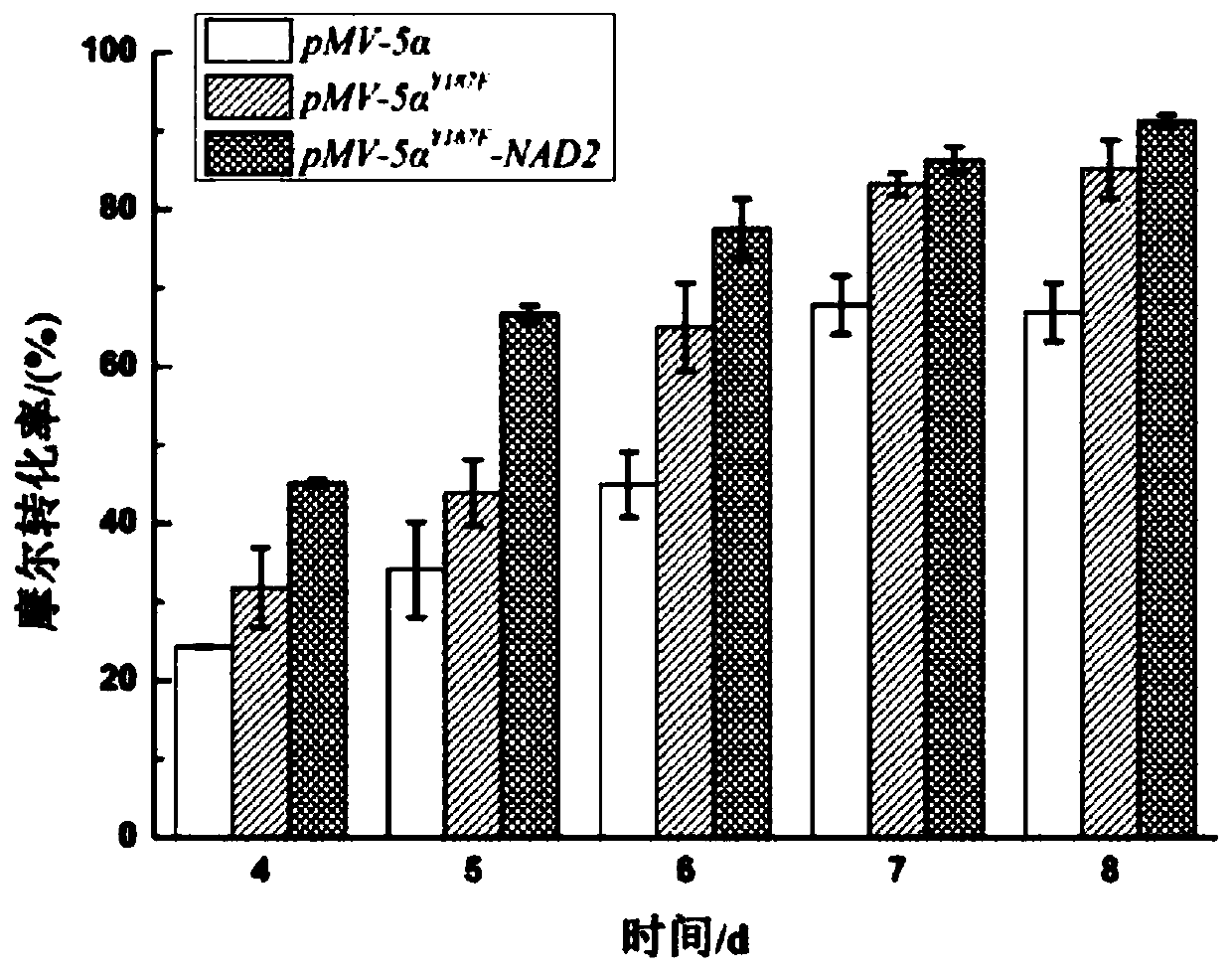
Construction of co-enzyme regeneration system and application of co-enzyme regeneration system in high-efficiency catalysis of 5 alpha-AD
A coenzyme regeneration system, -AD technology, applied in the field of genetic engineering, can solve problems such as unsatisfactory catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: Construction of mutant enzyme gene and recombinant expression vector
[0059]Using the synthetic recombinant plasmid pUC57-5α with the Treponema smegmatis 5α-reductase gene as a template, amplify the 5α-reductase gene whose nucleotide sequence is shown in SEQ ID NO.1, and amplify the amplified product It was connected with the Escherichia coli-mycobacterium shuttle plasmid pMV261, transformed into E.coli DH5α, and subjected to resistance screening, plasmid PCR, double enzyme digestion verification and DNA sequencing. The recombinant plasmid with correct sequencing and stable inheritance was selected and named pMV261-5α.
[0060] Using the pMV261-5α recombinant plasmid as a template, using F1 primer (sequence shown in SEQ ID NO.5) and R1primer (sequence shown in SEQ ID NO.6) as primers, site-directed mutagenesis was carried out by overlapping extension PCR to obtain The recombinant plasmid of the 5α-reductase mutant gene, named pMV261-5α Y187F .
[0061]...
Embodiment 2
[0063] Example 2: Recombinant bacterial strain MNR M3△ksdd / 261-5α Y187F build
[0064] The recombinant plasmid pMV261-5α obtained in Example 1 Y187F Electroporation to MNR M3△ksdd competent cells, screening strains that can be stably inherited is the 5α-reductase mutant mycobacterium engineering bacteria; the specific method is as follows:
[0065] 1) Take 10 μL of the plasmid that was sequenced correctly in the previous stage and add it to the melted MNR M3Δksdd competent cells, gently blow and mix with a gun, and treat in ice bath for about 15 minutes;
[0066] 2) Completely transfer the pre-cooled plasmid and competent cell mixed solution into a 1mm electric transfer cup (clean the electric transfer cup with absolute ethanol, place it on a sterile operating table, dry it, and place it on ice for pre-cooling for 5 minutes);
[0067] 3) Place the electroporation cup containing the mixture of plasmids and competent cells on a high-voltage pulse electroporation instrument, an...
Embodiment 3
[0071] Embodiment 3: Construction of recombinant bacterial strain MNR M3△ksdd / 261-5α
[0072] Use the 5α-reductase sequence shown in SEQ ID NO.1 as a template, and use the sequences shown in SEQ ID NO.9 and SEQ ID NO.10 as upstream and downstream primers to construct a recombinant strain MNR M3△ksdd / 261-5α, operate Process is the same as embodiment 1 embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com