A xylanase mutant with improved catalytic efficiency
A technology of xylanase mutation and carrier, which is applied in the fields of genetic engineering and protein expression, can solve the problems that need to be carried out and fail to reach industrial application, achieve great application potential, solve low catalytic activity, enzyme activity and kinetic parameters Improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Construction of embodiment 1 mutant library
[0024] By searching the protein PDB database, a xylanase crystal structure (PDB: 2VGD) with a protein sequence identity of 76% to xylanase AEx11A was obtained, and the xylanase AEx11A was analyzed using SWISS-MODEL with 2VGD as a template. Three-dimensional structure simulation, and molecular docking of the simulated AEx11A. Through the conservative analysis of the three-dimensional structure of AEx11A and the analysis of amino acid positions and physical and chemical properties, the 98th threonine was selected to be mutated into aspartic acid at the same time, and the 124th valine was mutated on the above basis. Saturation mutations were performed to screen out the optimal transformants. Based on the mutation sites designed above, design and synthesize specific site-directed mutagenesis primers T98D-F, T7-R (as shown in SEQ ID NO.5, SEQ ID NO.6) and saturation site-directed mutagenesis primer V124X-F (as shown in shown in...
Embodiment 2
[0026] Example 2: Screening of mutant libraries
[0027] The saturation mutation library pET-28a-AEx11A constructed in Example 1 T98D / V124X To transform E.coli BL21 competent cells, the specific method is as follows:
[0028] 1) Inoculate activated E.coli BL21 on LB plate in 2 mL LB medium, culture overnight at 37°C, 220r / min; inoculate 2% of the above culture solution in 5mL LB medium, culture at 37°C, 220r / min for 4h;
[0029] 2) Take 1.4mL of the above bacterial solution and put it into a 1.5mLEP tube, bathe in ice for 10min, centrifuge at 4000r / min for 2min, and collect the bacteria;
[0030] 3) Add 1mL of pre-cooled 0.1M CaCl 2 The above-mentioned cells were resuspended in the solution, placed in an ice bath for 10 minutes, centrifuged at 4000 r / min for 2 minutes, and the bacteria were collected;
[0031] 4) Add 100 μL of pre-cooled 0.1M CaCl 2 Suspend the above cells in the solution and store at 4°C for 30 minutes to transform;
[0032] 5) Take 100 μL of competent c...
Embodiment 3
[0039] The purification of embodiment 3 recombinant xylanase and the mensuration of enzymatic property
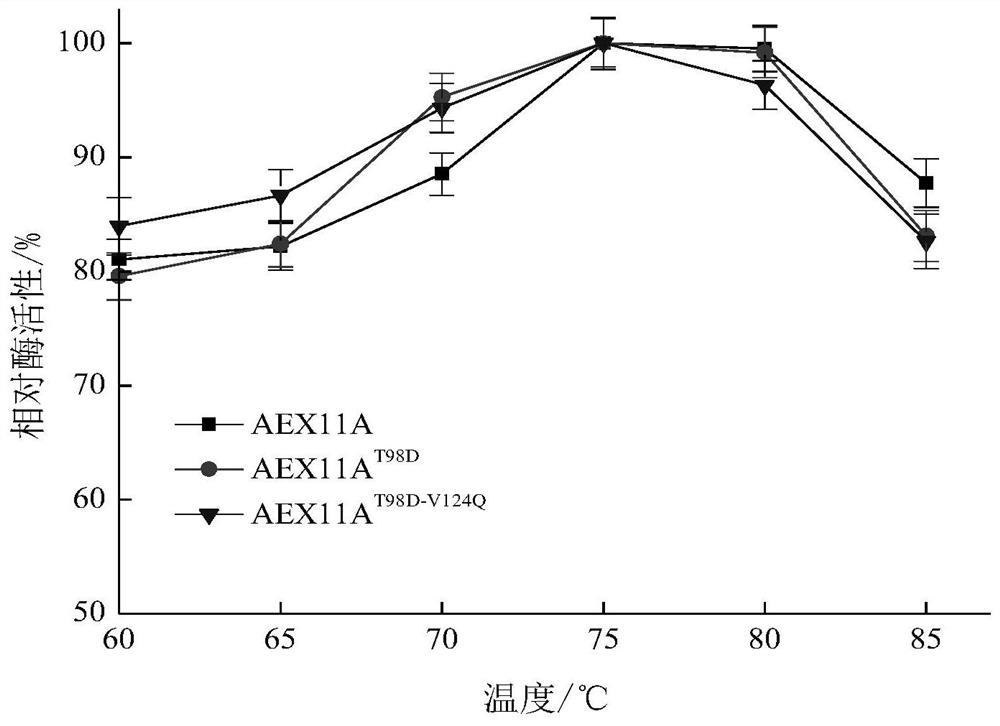
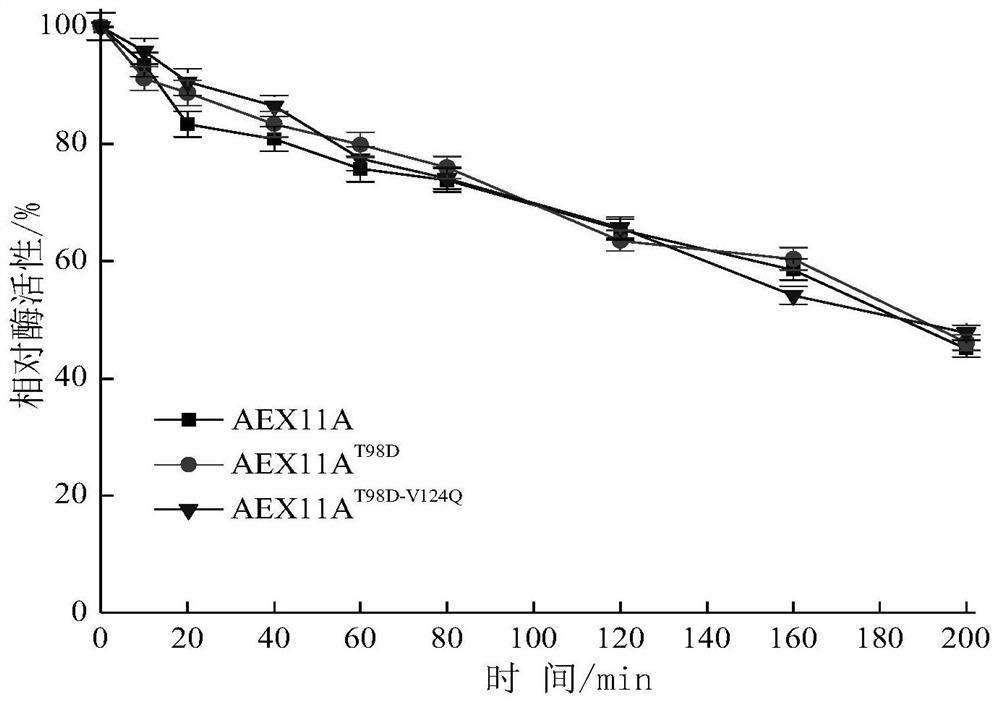
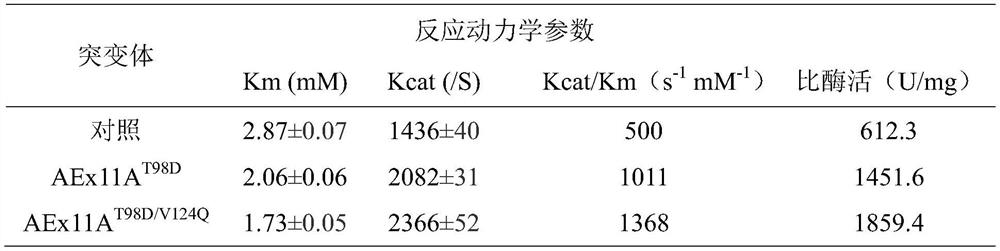
[0040] E.coli / AEx11A and E.coli / AEx11A T98D / V124Q The expression was induced by 0.4mmol / L IPTG at 20°C for 8h and the cells were disrupted by sonication, and the supernatant was purified by Ni-NAT column to purify the target enzyme protein. SDS-PAGE detection of purified AEx11A and AEx11A T98D / V124Q All present a single band at a relative molecular mass of 26.2kDa. For purified AEx11A and AEx11A T98D / V124Q Enzymatic property analysis, as shown in Table 1, AEx11A T98D / V124Q The specific activity of the mutant enzyme is 3.04 of that of AEx11A, and the catalytic efficiency is 2.74 times of that of AEx11A. Increased mutant AEx11A due to improved substrate affinity and catalytic efficiency T98D / V124Q specific enzyme activity. This shows that the invention improves the enzymatic properties of xylanase through the innovative way of mutation.
[0041] The relative activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com