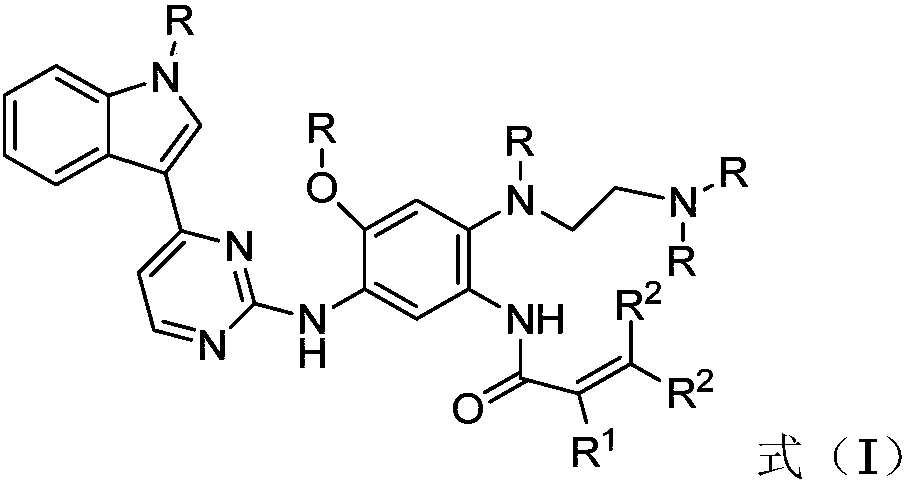
2-(2,4,5-substituted phenylamino)pyrimidine derivative as well as preparation method and application thereof to preparation of anti-tumor drug
A technology of pyrimidine derivatives and anti-tumor drugs, which is applied in the field of medicine, can solve the problems of lack of allylamine modification, and achieve the effects of high selectivity, inhibitory activity and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1. Preparation of intermediate compound (1):
[0023]
[0024] Intermediate Compound (1) was prepared according to the method disclosed in International Patent WO 2013 / 01448. Its derivatives can also be prepared in a similar manner.
Embodiment 2
[0025] Example 2. Preparation of compound (III) and analogs:
[0026]
[0027] 187mg, (2mmol) CH 2 Cl 2 (5mL) solution was added to CH containing compound 1 (802mg, 1.8mmol) and N,N-diisopropylethylamine (DIPEA) (0.14mL, 4.2mmol) at 0°C 2 Cl 2 (5 mL) solution. The mixture was stirred at room temperature for 2 hours, washed with water, Na 2 SO 4 After drying, the solvent was evaporated and the residue was separated by silica gel column chromatography (0-10% 7N NH 3 MeOH / CH 2 Cl 2 ) to obtain compound III (754 mg, yield 83%). H-NMR (DMSO-d 6 ):2.22(6H,s),2.28(2H,t),2.73(3H,s),2.87(2H,t),3.85(3H,s),3.93(3H,s), 7.02(1H,s) ,7.16(1H,t),7.21-7.26(2H,m),7.52(1H,d),7.92(1H,s),8.23(1H,d),8.34(1H,d),8.67(1H,s ),9.12(1H,s),10.23(1H,s).m / z:503ES + [M+H] + ..
[0028] Using the same method as in Example 1, the following corresponding products were prepared with differently substituted acryloyl chlorides: compound (IV):
[0029]
[0030] Yield 81%. H-NMR (DMSO-d 6 ):2.2...
Embodiment 4
[0043] Embodiment 4. the mesylate salt (X) of preparation compound III
[0044]
[0045] Compound III (5.03g, 10mmol) was added to a 100mL flask containing EtOH (30mL) and EtOAc (20mL), and a solution of methanesulfonic acid (1.12g, 10mmol) in EtOAc (10mL) was added dropwise at room temperature within 30 minutes. After the addition was complete, the mixture was stirred at room temperature for 2 hours. After filtration, the filter cake was washed once with EtOAc / EtOH (2:1, v / v), and dried to obtain a yellow solid X (4.9 g, yield 80%).
[0046] The preparation of the mesylate (XI) of compound IV is the same as above: compound IV and methanesulfonic acid generate yellow solid XI with a yield of 85%.
[0047]
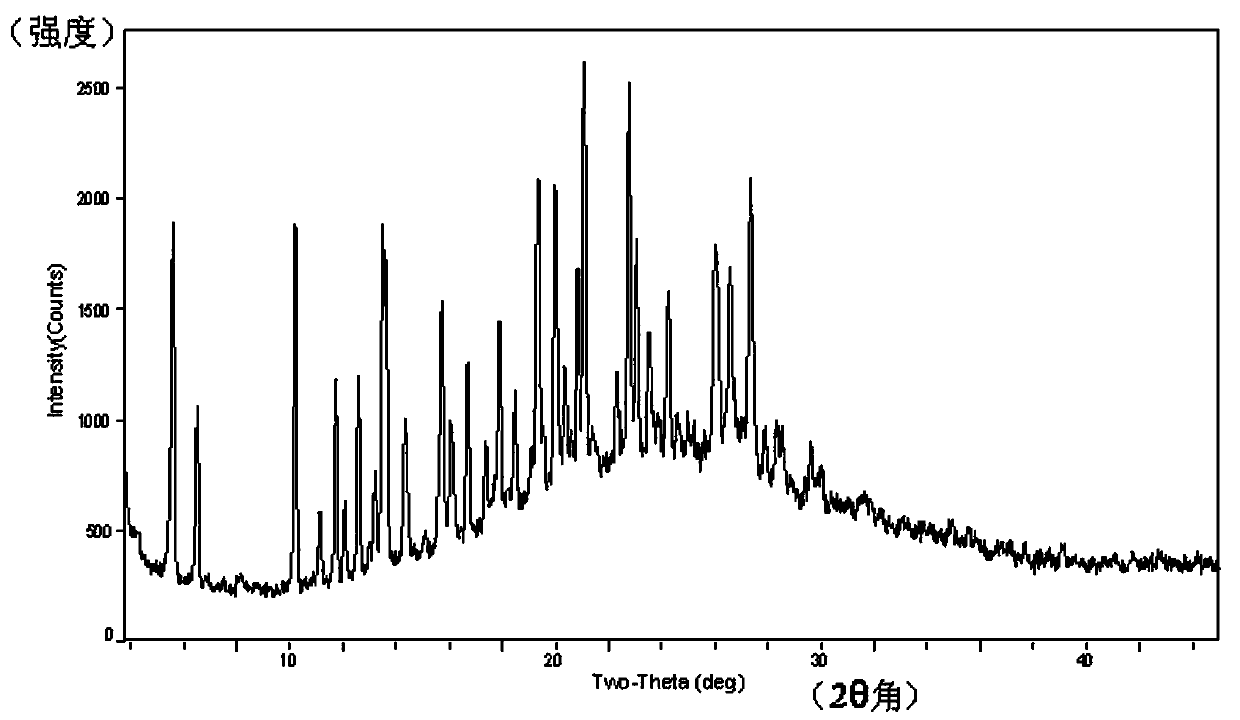
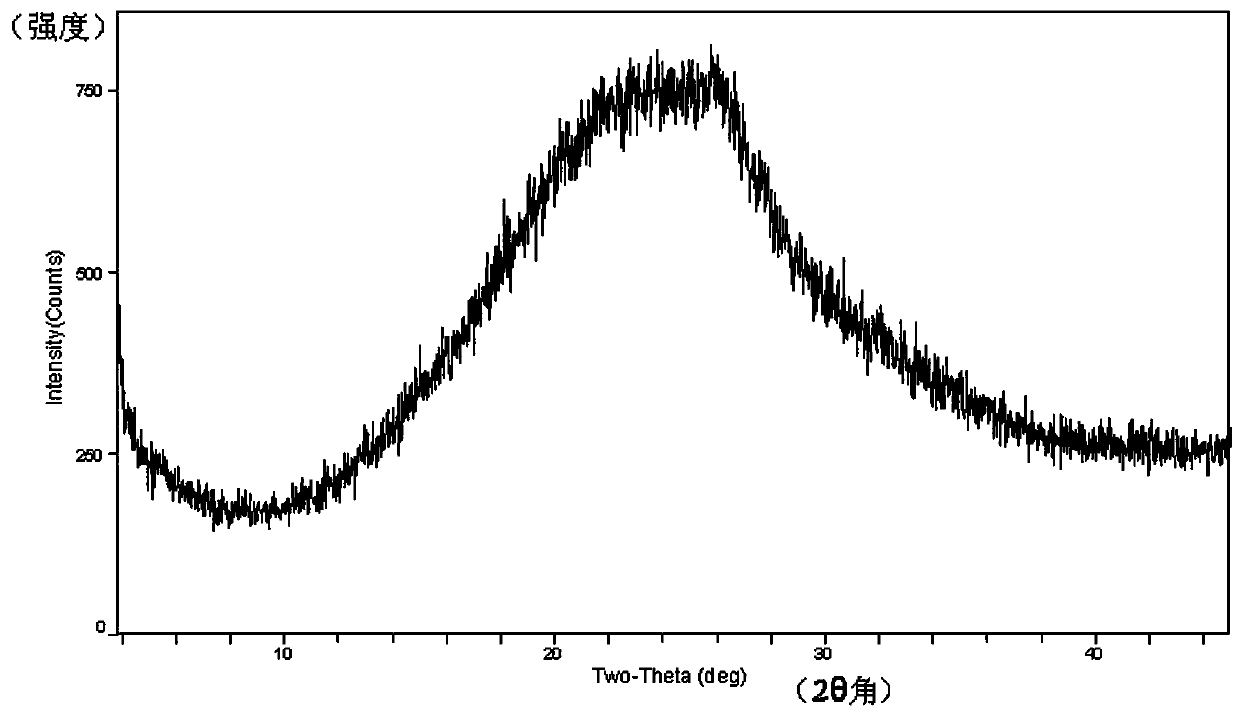
[0048] The X-ray powder diffraction of the crystalline form of the compound of formula XI has characteristic peaks and their relative intensities (%) at the following diffraction angles 2θ:
[0049]
[0050] X-ray powder diffraction (XRPD) of compound XI crystal:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com