Method for synthesizing 2, 3-dimethyl-4-fluorophenol
A technology of dimethyl fluorobenzene and fluorophenol is applied in the field of synthesis of fluorine-containing compounds and achieves the effects of cost reduction, easy availability of raw materials and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
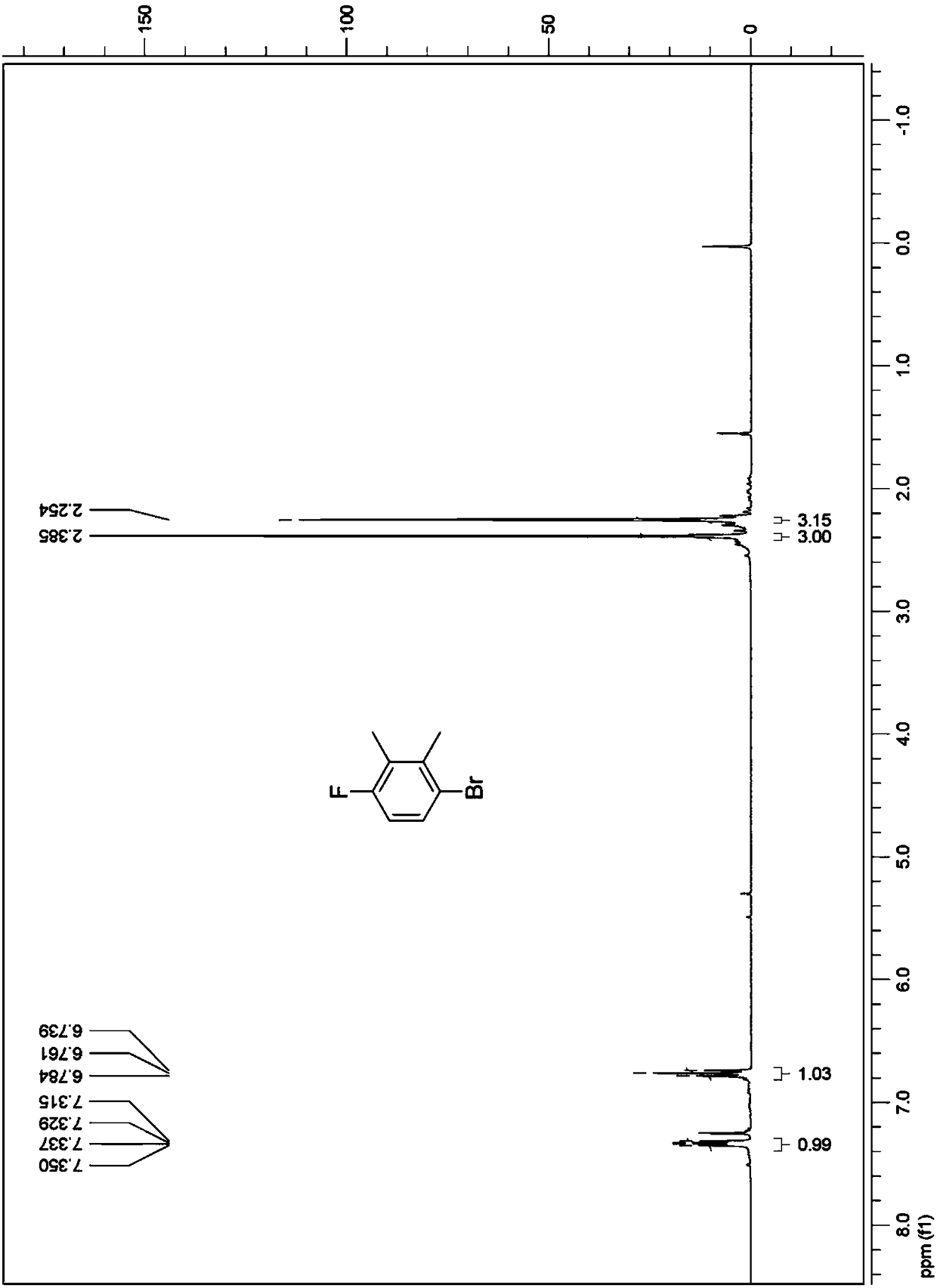
[0064] (1) Preparation of 3-bromo-6-fluoro-o-xylene
[0065] Add 400g of dichloromethane, 124g of 2,3-dimethylfluorobenzene, and 5g of aluminum trichloride into a 1L three-necked reaction flask in sequence, and stir at 20°C; from a constant pressure dropping funnel containing 160g of bromine Release 16-32g of bromine at a time and add it to the three-necked reaction flask, stir at 30°C, trigger the reaction and generate a large amount of hydrogen bromide to make the reaction liquid red; at this time, add the remaining bromine in the constant pressure dropping funnel dropwise to In the three-necked reaction flask, stir at 20° C., and the reaction ends after 0.5 hour of dropwise addition.
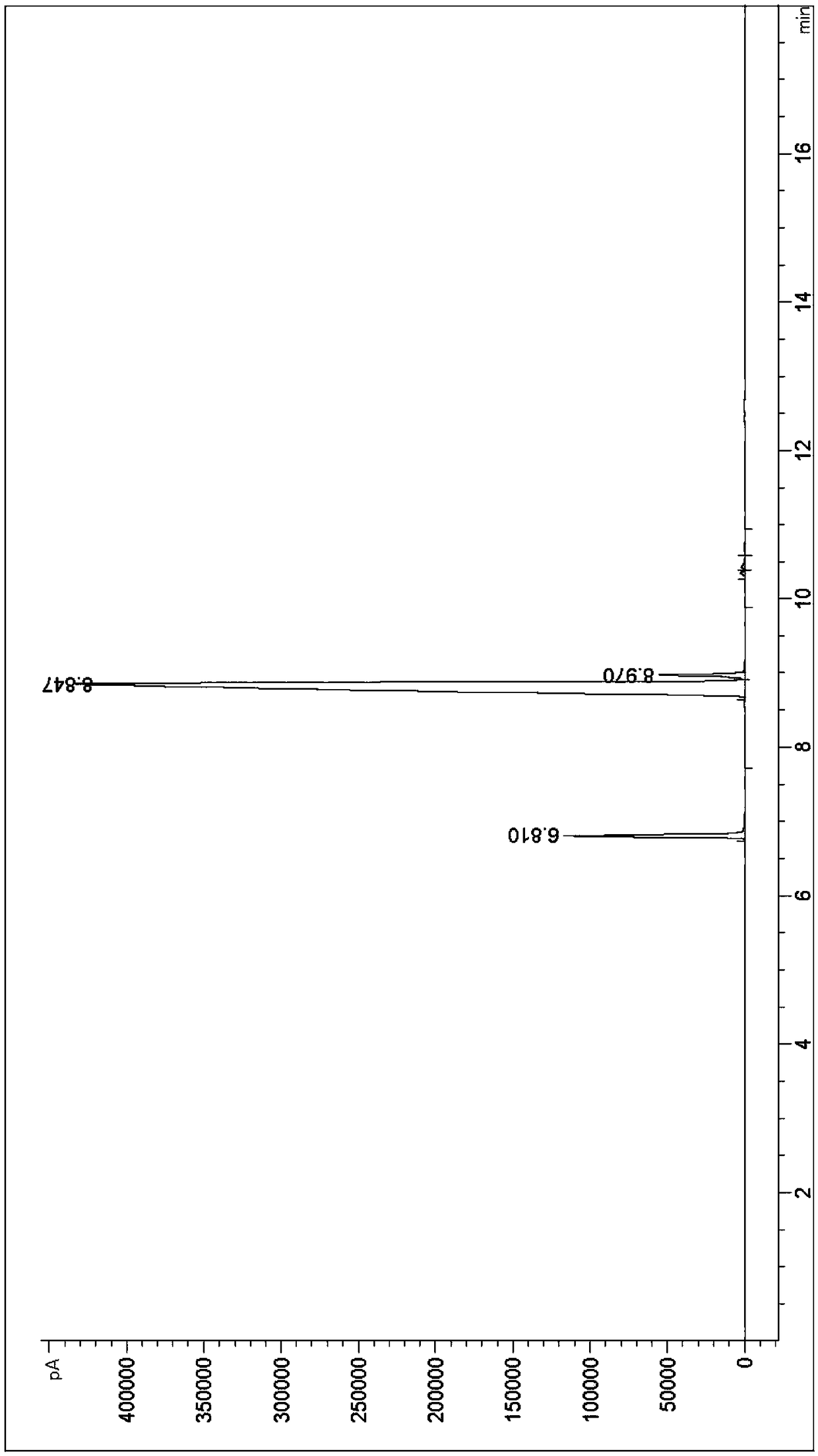
[0066] After the reaction, the reaction solution was post-treated: 50ml of saturated sodium sulfite aqueous solution was added dropwise to the reaction solution in the there-necked flask to remove a small amount of unreacted bromine. At room temperature, wait until the reaction solution gradu...
Embodiment 2
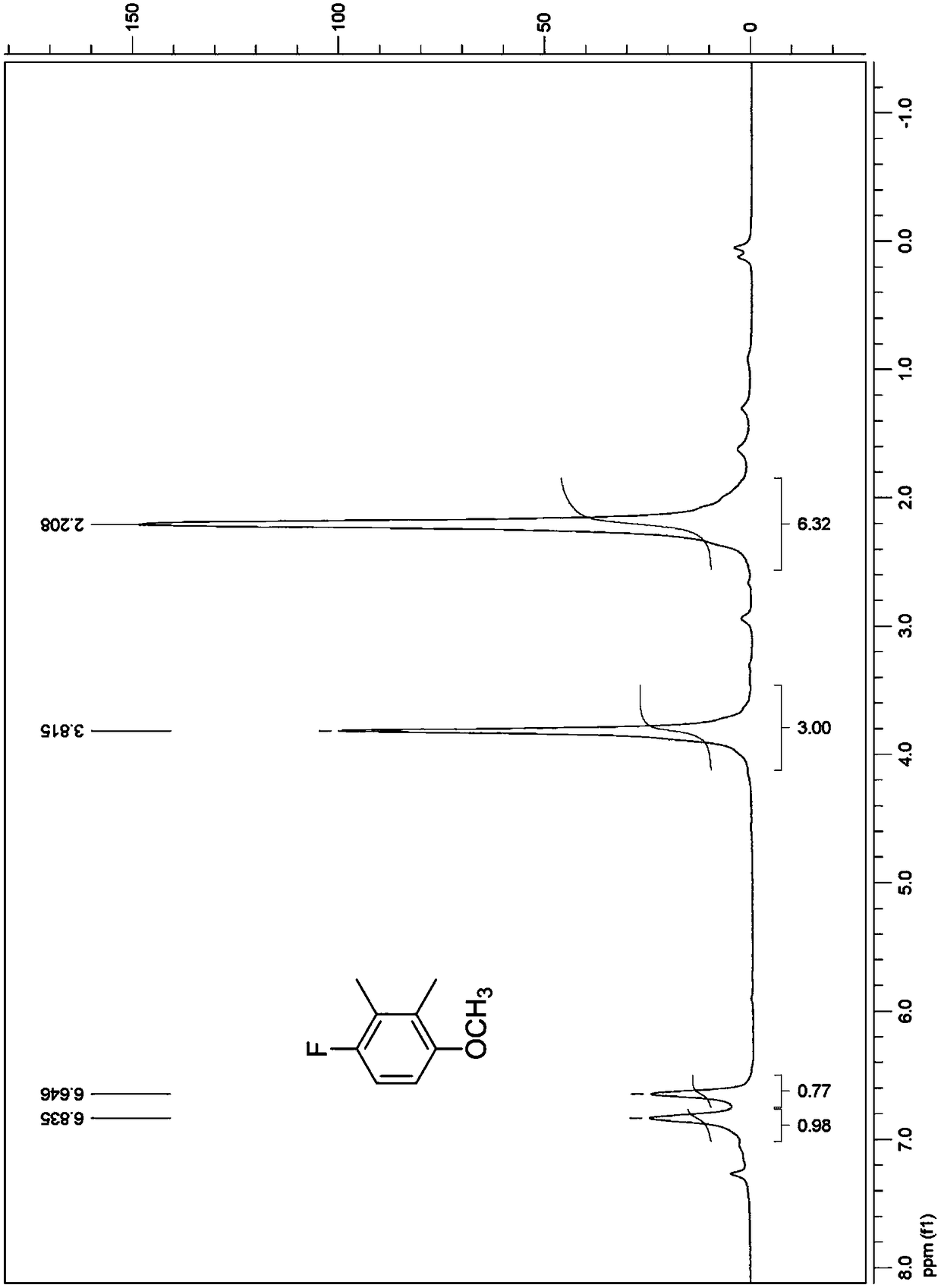
[0084] (1) Preparation of 3-bromo-6-fluoro-o-xylene
[0085]Add 800g of dichloromethane, 372g of 2,3-dimethylfluorobenzene, and 15g of aluminum trichloride into a 3L three-necked reaction flask in sequence, and stir at 25°C; Emit 48~96g (10%~20% of total amount of bromine) bromine once and add into three-necked reaction bottle, stir at 35 ℃, initiate reaction and produce a large amount of hydrogen bromide and make reaction liquid be red; The remaining bromine in the dropping funnel was added dropwise into the three-necked reaction flask, stirred at 25°C, and the reaction was completed for 1 hour after the dropwise addition.
[0086] After the reaction, the reaction solution is post-treated: 150ml of saturated sodium sulfite aqueous solution is added dropwise to the reaction solution in the there-necked flask to remove a small amount of unreacted bromine. At room temperature, wait until the reaction solution gradually becomes colorless, which means The bromine treatment is com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com