CO2 enriched type two-phase absorbent with rapid and spontaneous layering
An enrichment, absorbent technology, applied in separation methods, dispersed particle separation, gas treatment, etc., can solve the problems of small load range, limited use, and high investment in phase separation, and achieve lower capture energy consumption and thermal stability. High, low volatility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of absorbent: Measure 50g of diethylaminoethanol (DEEA), 25g of ethanolamine (MEA) and 25g of deionized water, mix evenly, and prepare 50wt.% DEEA+25wt.%MEA+25wt.%H 2 Absorbent solution 100g of O, stand for later use.
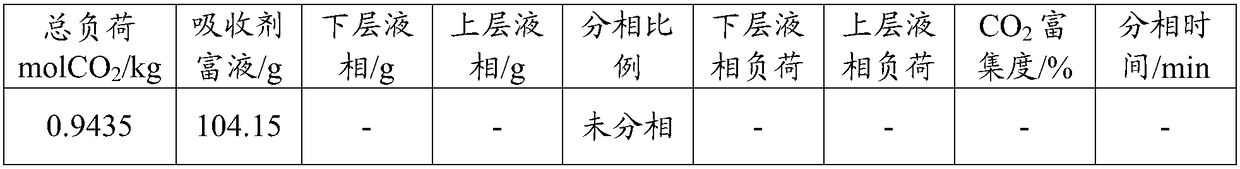
[0030] Then, absorb CO at a temperature of 40°C and normal pressure 2 , at different CO 2 Under load, phase separation ratio of absorbent rich liquid, CO 2 load distribution, CO 2 The enrichment degree and phase separation time are shown in Table 1 below.
[0031] The load in the table is defined as: CO absorbed per kg of amine and water in the absorbent 2 The amount of mol; the phase split ratio is defined as: absorbent rich liquid absorbs CO 2 After phase separation, the mass percentage of the lower liquid phase in the total liquid phase; CO 2 The degree of enrichment is defined as: CO absorbed in the lower liquid phase 2 % of total liquid phase absorbed CO 2 The mole percentage.
[0032] Table 1 is the different CO of the absorbent ...
Embodiment 2
[0037] Preparation of absorbent: Measure 50g of diethylaminoethanol (DEEA), 25g of hydroxyethylethylenediamine (AEEA) and 25g of deionized water, mix evenly, and prepare 50wt.% DEEA+25wt.% AEEA+25wt.% h 2 Absorbent solution 100g of O, stand for later use.
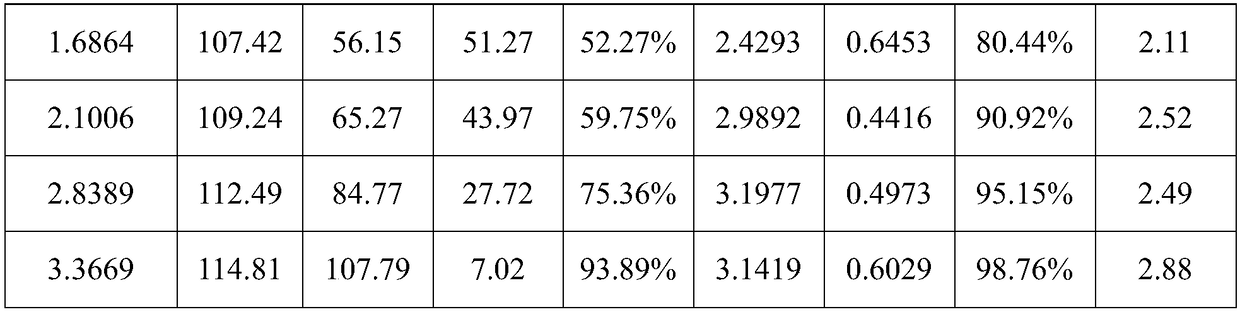
[0038] Then, absorb CO at a temperature of 40°C and normal pressure 2 , at different CO 2 Under load, phase separation ratio of absorbent rich liquid, CO 2 load distribution, CO 2 The enrichment degree and phase separation time are shown in Table 2 below.
[0039] Table 2 is the different CO of the absorbent synthesized in embodiment 2 2 Performance parameters under load
[0040]
[0041] It can be seen from Table 2 that the phase separation load of the two-phase absorbent in this example to spontaneously form a liquid-liquid two-phase is 1.30-2.79mol CO 2 / kg absorbent.
Embodiment 3
[0043] Absorbent preparation: measure 50g diethylaminoethanol (DEEA), 20g hydroxyethylethylenediamine (AEEA), 5g piperazine (PZ) and 25g deionized water respectively, mix them evenly, and prepare 50wt.% DEEA+20wt .%AEEA+5wt.%PZ+25wt.%H 2 Absorbent solution 100g of O, stand for later use.
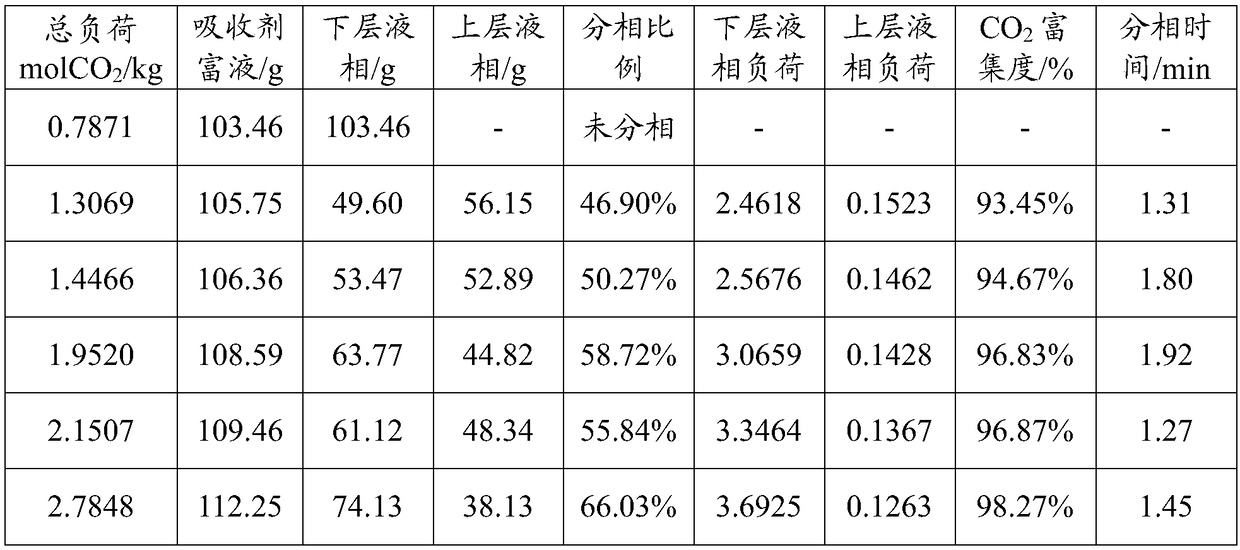
[0044] Then, absorb CO at a temperature of 40°C and normal pressure 2 , at different CO 2 Under load, phase separation ratio of absorbent rich liquid, CO 2 load distribution, CO 2 The enrichment degree and phase separation time are shown in Table 3 below.
[0045] Table 3 is the different CO of the absorbent synthesized in embodiment 3 2 Performance parameters under load
[0046]
[0047] It can be seen from Table 3 that the phase separation load of the two-phase absorbent in this example to spontaneously form a liquid-liquid two-phase is 1.70-3.33mol CO 2 / kg absorbent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com