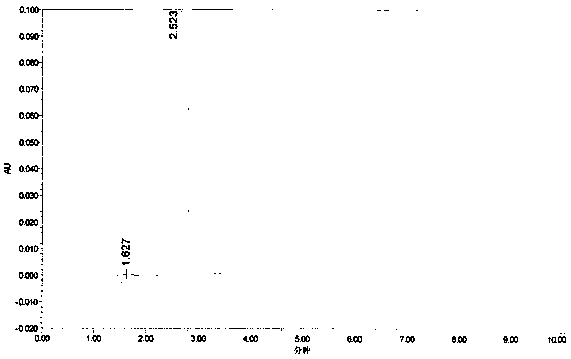
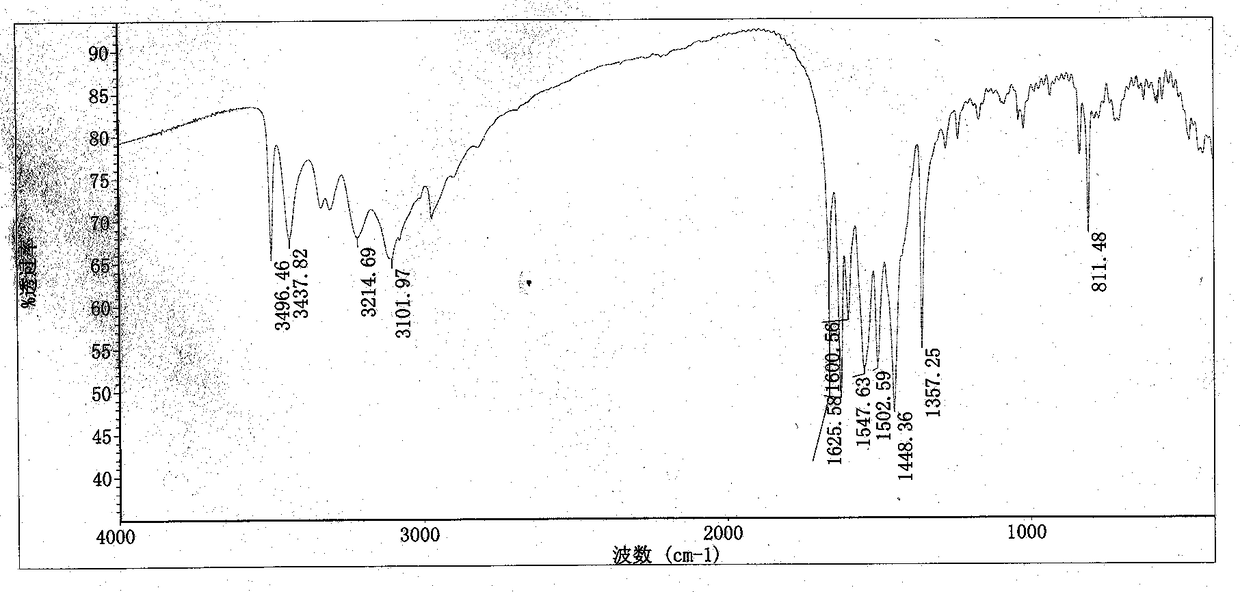
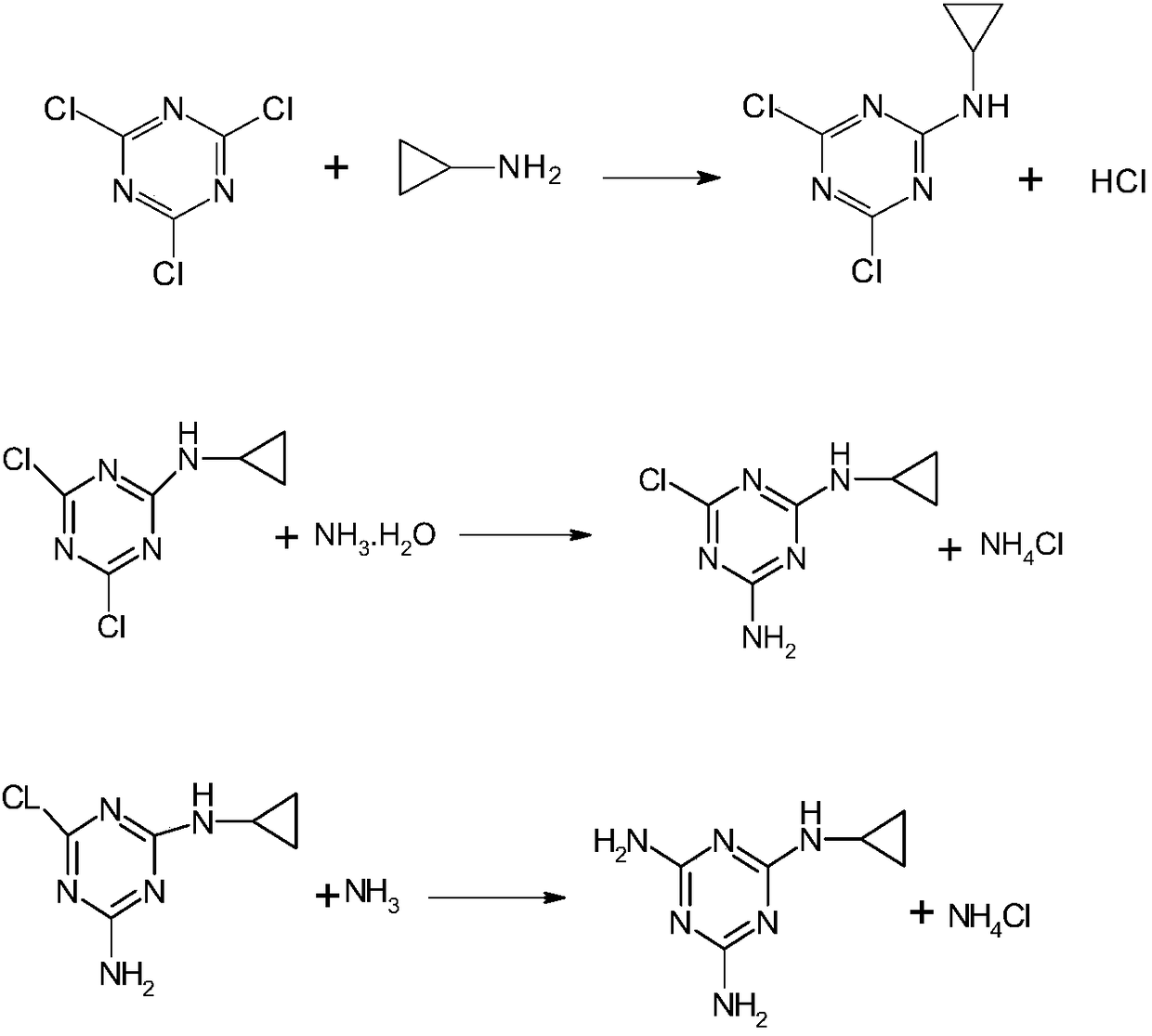
Method for preparing cyromazine
A technology of cyromazine and ammonia water, which is applied in the field of veterinary drugs and pesticides, can solve the problems of high risk factor, hidden safety hazard, flammability and explosion, etc., and achieve the effect of high safety factor, pollution avoidance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for cyromazine, comprising the following steps:
[0032] 1) Dissolve 30g of cyanuric chloride in 155g of trichlorethylene at 0°C under stirring conditions, and add dropwise 60g of 25% industrial ammonia water; heat up to 40°C, react for 6 hours to obtain a reaction solution, and under the same temperature conditions , after separating trichlorethylene by vacuum distillation, add 180g of purified water, stir for 1 hour, then lower to 25°C, filter with suction, wash with water, and dry in vacuum for 8h at 50°C to obtain 22.5g of intermediate, which is 2-chloro -4.6-diamino-1.3.5-triazine dry product, the mass yield of the intermediate is 95%;
[0033] 2) Add 165ml of purified water to 22.5g of the intermediate in step 1, then add 10.5g of cyclopropylamine, heat up to 90°C, add dropwise an aqueous solution of sodium carbonate (an aqueous solution prepared by dissolving 8.8g of sodium carbonate in 27ml of purified water), Adjust pH=7.5-8.5, keep warm f...
Embodiment 2
[0035] A preparation method for cyromazine, comprising the following steps:
[0036] 1) Dissolve 20g of cyanuric chloride in 120g of 1,2-dichloroethane at 5°C under stirring condition, add dropwise 40g of 25% industrial ammonia water; heat up to 45°C, react for 6h to obtain a reaction solution, Under the same temperature conditions, after vacuum distillation and separation of 1,2-dichloroethane, 120g of purified water was added, stirred for 1 hour, then lowered to 25°C, filtered with suction, washed with water, and vacuum-dried at 55°C for 6h to obtain 14.8g Intermediate, the intermediate is a dry product of 2-chloro-4.6-diamino-1.3.5-triazine, and the mass yield of the intermediate is 94%;
[0037] 2) Add 120ml of purified water to the intermediate of step 1), then add 7.2g of cyclopropylamine, heat up to 95°C, add dropwise an aqueous solution of potassium carbonate (an aqueous solution prepared by dissolving 7.8g of potassium carbonate in 28ml of purified water), and adjust ...
Embodiment 3
[0039] A preparation method for cyromazine, comprising the following steps:
[0040] 1) Dissolve 30 g of cyanuric chloride in 160 g of 1,2-dichloroethane at 3° C. under stirring, and dropwise add 60 g of 25% industrial ammonia water; raise the temperature to 42° C., and react for 6 hours to obtain a reaction solution. Under the same temperature conditions, after vacuum distillation and separation of 1,2-dichloroethane, 180g of purified water was added, stirred for 1 hour, then lowered to 25°C, suction filtered, washed with water, and vacuum-dried at 52°C for 7h to obtain 22.8g Intermediate (dry product of 2-chloro-4.6-diamino-1.3.5-triazine), the mass yield of the intermediate is 96%;
[0041] 2) Add 170ml of purified water to the intermediate in step 1), then add 10.8g of cyclopropylamine, heat up to 92°C, add dropwise an aqueous solution of potassium carbonate (an aqueous solution prepared by dissolving 9.0g of potassium carbonate in 30ml of purified water), Adjust pH=7.5-8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com