Preparation method of mezlocillin sodium-sulbactam sodium for injection
A technology of mezlocillin sodium sulbactam sodium and mezlocillin sodium, applied in the field of medicine, can solve the problems of stratification, single product, unable to meet diversified requirements, etc., and achieve the effect of good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
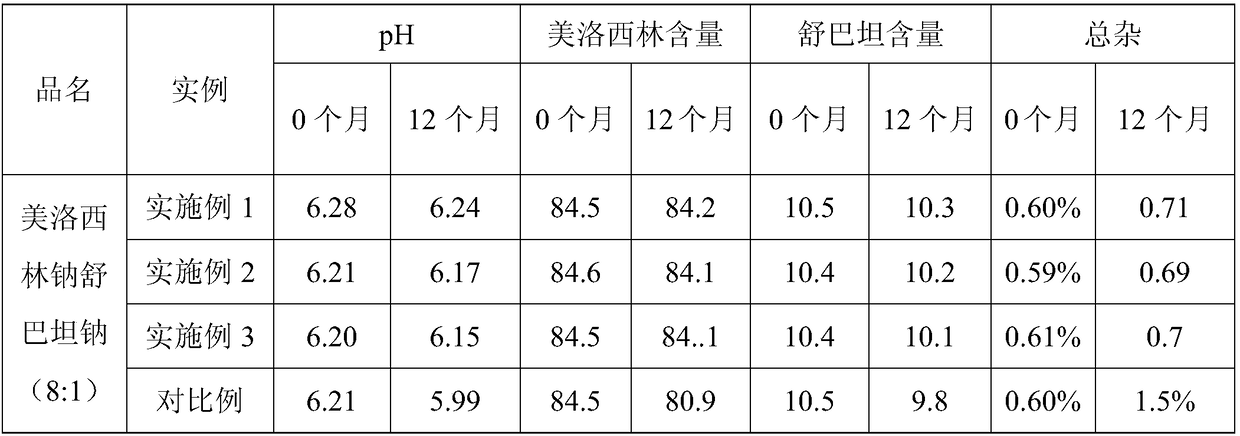
[0022] Example 1: Freeze-dried preparation of mezlocillin sodium and sulbactam sodium for injection (8:1)
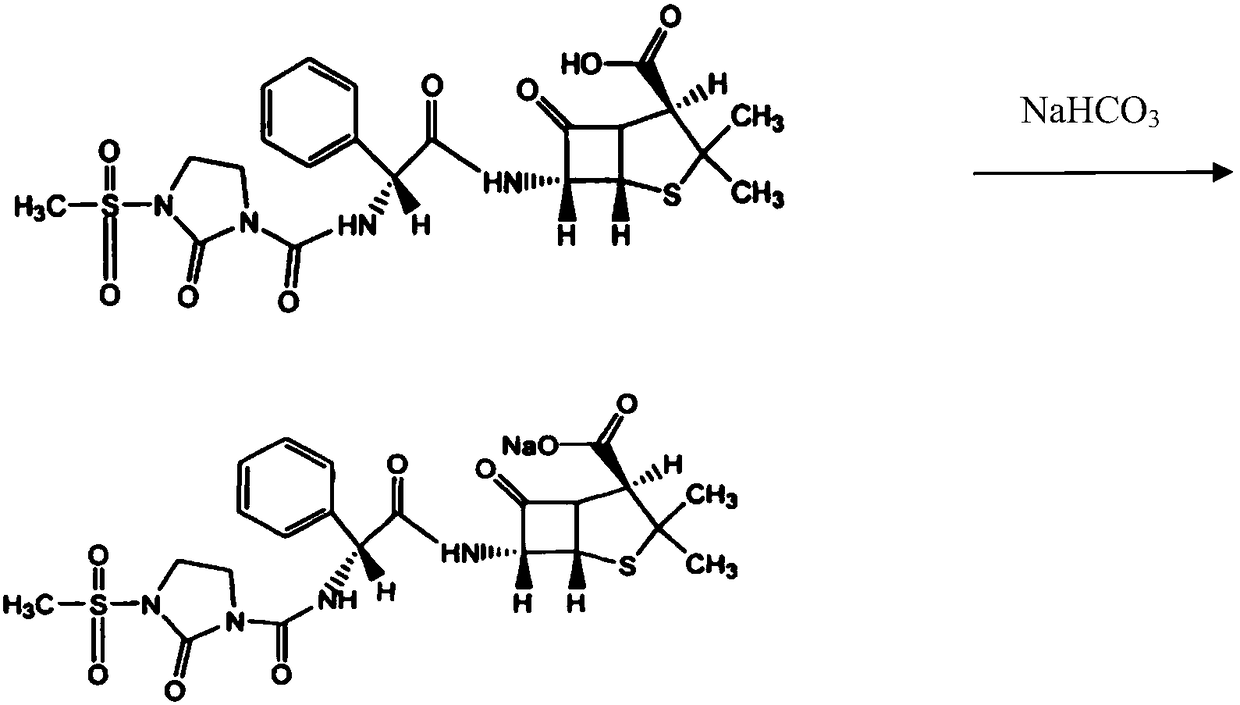
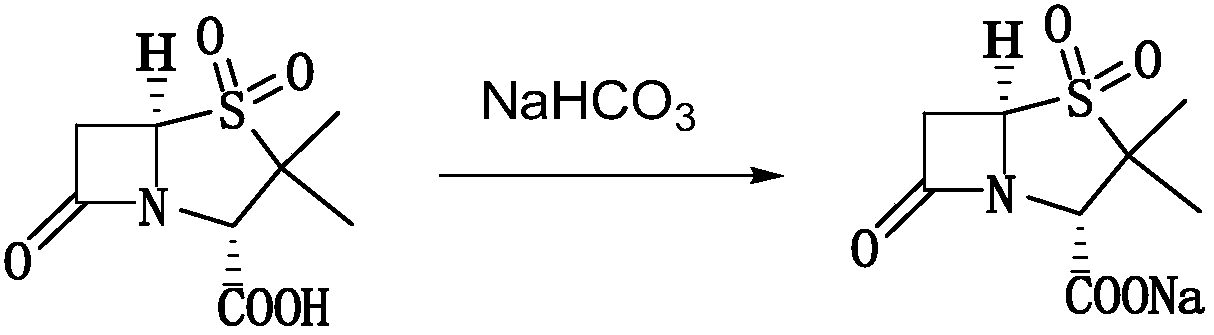
[0023] 1) 100ml of bottom water is added in the batching tank, cooled to 8.9°C, 124.7g mezlocillin is transferred into the batching tank, and the 14% sodium bicarbonate solution (19.36g sodium bicarbonate is added with water to be mixed with 14wt% Sodium bicarbonate solution) is added dropwise into the batching tank, and the temperature is controlled at 5-10°C. Pay attention to observe the reaction in the tank. If there are too many foams, slow down the feeding speed to prevent rushing. Observe the online pH value at any time to control the feeding process The pH should not be higher than 7.0.
[0024] Continue to add 14.7g of sulbactam, while the prepared 14wt% sodium bicarbonate solution (4.10g sodium bicarbonate is added with water to be mixed with 14wt% sodium bicarbonate solution) is added dropwise in the batching tank, and the control batching tank is added in the ...
Embodiment 2
[0030] Mezlocillin: moisture 3.4%, content 99.2%, weighing 124.7g;
[0031] Sulbactam: moisture 0.04%, content 99.9%, weighing 14.7g;
[0032] Sodium bicarbonate: 23.46g (19.36g+4.10g, prepared as a solution with a concentration of 14wt%).
[0033] EDTA: 31.06mg.
[0034] 1) Bottom water 110ml, cool down to 7.5°C, add 124.7g of mezlocillin, 14.7g of sulbactam, dropwise add sodium bicarbonate: 23.46g;
[0035] 2) Add the EDTA solution dissolved in advance, after adding the EDTA solution, continue to stir for 20 minutes, and then vacuum again;
[0036] 3) After 1 hour the pH is 6.21, ready for filtration.
[0037] 4) Sterilize, filter, and freeze-dry to obtain the original powder of mezlocillin sodium and sulbactam sodium (8:1).
[0038] All the other are with embodiment 1.
Embodiment 3
[0040] Mezlocillin: moisture 3.4%, content 99.2%, weighing 124.7g;
[0041] Sulbactam: moisture 0.04%, content 99.9%, weighing 14.7g;
[0042] Sodium bicarbonate: 23.46g (19.36g+4.10g, prepared as a solution with a concentration of 15wt%);
[0043] EDTA: 31.06mg.
[0044] 1) Bottom water 115ml, cool down to 6.3°C, add 124.7g of mezlocillin, 14.7g of sulbactam, dropwise add sodium bicarbonate: 23.46g;
[0045] 2) Add the EDTA solution dissolved in advance, after adding the EDTA solution, continue to stir for 20 minutes, and then vacuum again;
[0046] 3) After 1 hour, the pH is 6.20, ready for filtration;
[0047] 4) Sterilize, filter, and freeze-dry to obtain the original powder of mezlocillin sodium and sulbactam sodium (8:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com