Production method of tazobactam sodium for injection
A technology of tazobactam sodium and a production method, which is applied in the field of medicine, can solve the problems of slow drying speed, prolonged production cycle, large resistance to water sublimation, etc., and achieves the effects of increasing acidity stability and good grain consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
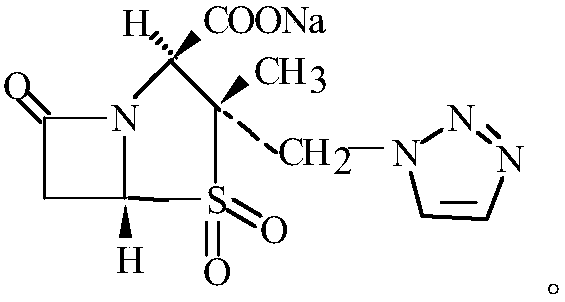
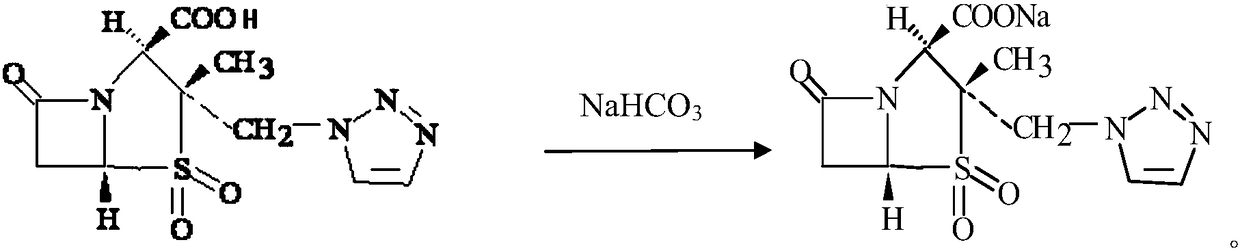
[0035] 1. Salt formation
[0036] Raw materials and dosage: 132kg of tazobactam, 36.8kg of sodium bicarbonate.
[0037] ① Add 200ml of bottom water to the reaction tank, turn on the interlayer refrigerant water to cool down, and turn off the refrigerant water when the temperature drops to 5-10°C.
[0038] Then the sodium bicarbonate 36.8g that has weighed is added water and is mixed with the sodium hydroxide solution that concentration is 25% (w / v) for subsequent use, reserves the sodium bicarbonate solution to adjust the pH value usefulness, and all the other sodium bicarbonate solutions are ready to be added dropwise .
[0039] ② Put the weighed tazobactam into the reaction tank, and start to drop the prepared lye at the same time, keep the lye in a suspension state without precipitation during the alkali addition process, and maintain the temperature in the reaction tank at 5 -10°C.
[0040] ③ Turn on the vacuum to remove carbon dioxide gas.
[0041] ④Adjust the pH to 6...
Embodiment 2
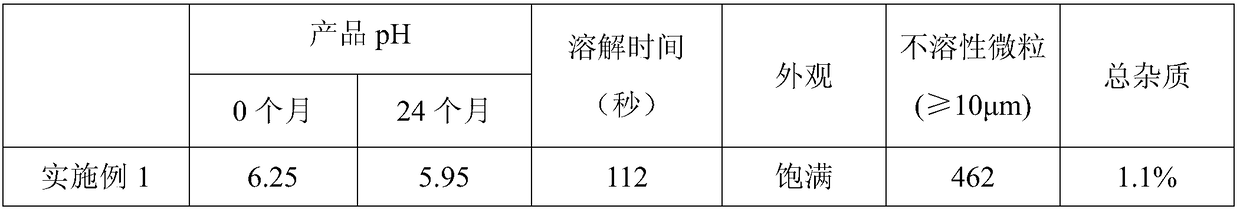
[0055] After step ④ is completed, add 38 mg of EDTA solution and stir for 20 minutes, then re-evacuate the carbon dioxide gas and maintain the pH at 6.0-6.5.
[0056] All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com