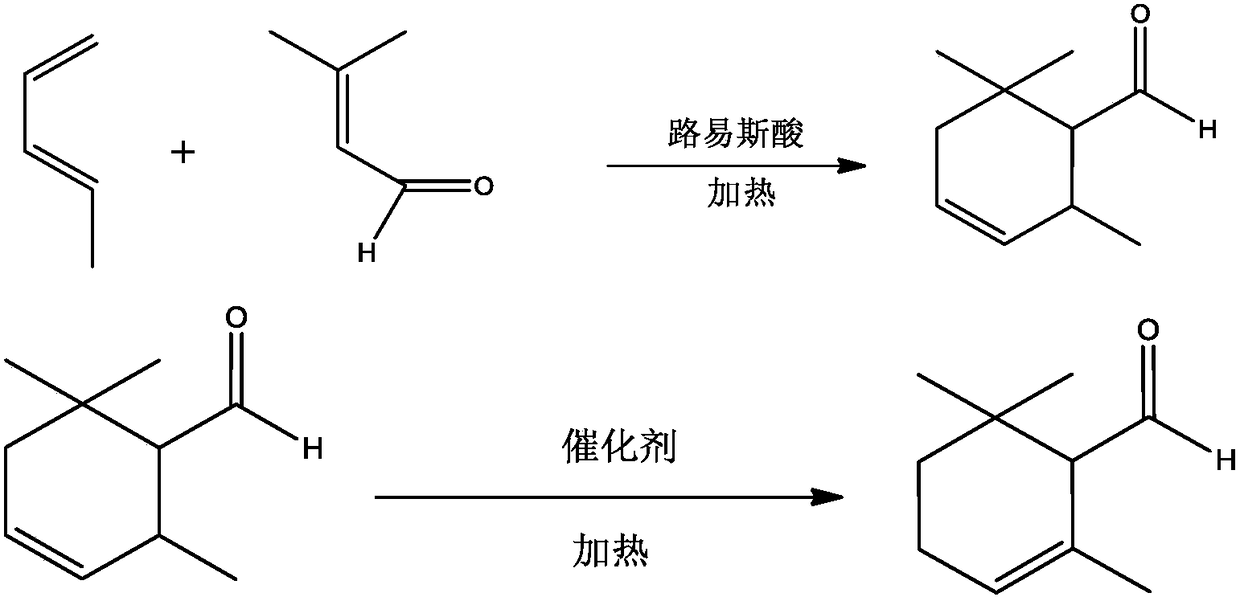
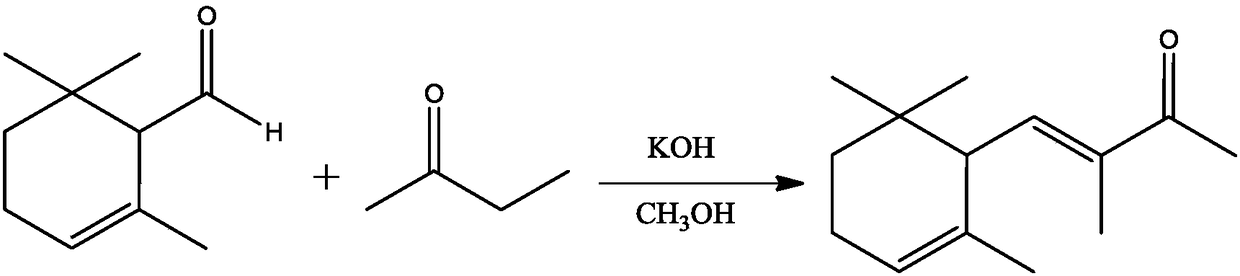
Synthesis method of alpha-cyclocitral
The technology of a cyclocitral and a synthesis method is applied in the directions of chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., and can solve the problems of unsatisfactory aroma, many beta isomers, large equipment investment, and the like, Achieve the effect of no by-products, high selectivity and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1) Add 84.12g of 1,3-pentadiene, 80.00g of prenal, and 14.67g of aluminum trichloride under the protection of nitrogen into the reaction kettle that has undergone dehydration and deoxidation treatment, and stir and react at 90°C for 1.0h; The temperature was lowered to room temperature, and 180.00 g of crushed ice was poured into the reaction solution, stirred thoroughly for 10 minutes, and then moved to a separatory funnel for static separation to obtain 184.66 g of the aqueous phase and 174.13 g of the organic phase; the organic phase was transferred to the reaction kettle, NMR qualitative, the data are as follows: 1 H-NMR (δ, ppm, 400MHz, CDCl 3 ):0.99(s,3H,-CH 3 ),0.99(s,3H,-CH 3 ),1.75-2.00(m,2H,-CH 2 ),1.11(m,3H,=C-CH 3 ), 1.62~1.69(m, 2H, -CH=CH-), 2.48(d, J=8.0Hz, 1H, -CH-CHO), 5.73(t, J=2.8Hz, 1H, -CH=), 9.72 (s, 1H, -CHO); 13C-NMR (400MHz, CDCl 3 )δ (ppm): 202.3; 131.3; 124.8; 71.8; 40.535.2; 28.2; 27.7; 27.7; Add 2.16g of nickel chloride, 1.42g of zinc...
Embodiment 2
[0055] In the reaction kettle that has been dehydrated and deoxygenated, 84.12g of 1,3-pentadiene, 80.00g of prenaldehyde and 14.67g of aluminum trichloride were added under the protection of nitrogen, and the reaction was stirred at 80°C for 1.0h; the temperature dropped to room temperature, poured 180.00 g of crushed ice into the reaction solution, stirred thoroughly for 10 min, and then moved to a separatory funnel for static separation to obtain 184.20 g of the aqueous phase and 175.23 g of the organic phase; Add 2.17g nickel chloride, 1.43g zinc powder, 0.05g ammonium chloride, 19.83g triphenylphosphine and 200.00g toluene, stir and react at 55°C for 1.7h, filter the reaction solution after cooling down, and remove toluene by rotary evaporation , the organic phase was distilled under reduced pressure at 1.86kPa, and the fractions at 84° C. to 92° C. were collected to obtain 158.38 g of pure α-cyclic citral. The gas chromatography analysis selectivity was 99.80%, and the yi...
Embodiment 3
[0057] In the reaction kettle that has been dehydrated and deoxygenated, 84.12g of 1,3-pentadiene, 80.00g of prenaldehyde and 14.67g of aluminum trichloride were added under the protection of nitrogen, and the reaction was stirred at 100°C for 1.0h; the temperature dropped to room temperature, poured 180.00 g of crushed ice into the reaction solution, stirred thoroughly for 10 min, and then moved to a separatory funnel for static separation to obtain 184.10 g of the aqueous phase and 175.00 g of the organic phase; Add 2.17g nickel chloride, 1.43g zinc powder, 0.05g ammonium chloride, 19.80g triphenylphosphine and 200.00g toluene, stir and react at 55°C for 1.7h, filter the reaction solution after cooling down, and remove toluene by rotary evaporation , the organic phase was distilled under reduced pressure at 1.86kPa, and the fractions at 84°C to 92°C were collected to obtain 156.57g of pure α-cyclocitral, with a gas chromatography selectivity of 99.80% and a yield of 95.40%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com