Medicament for gene therapy of diabetes type I
A technology of gene therapy and gene expression, applied in the field of gene therapy drugs for type 1 diabetes, which can solve the problems of reducing the probability of immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
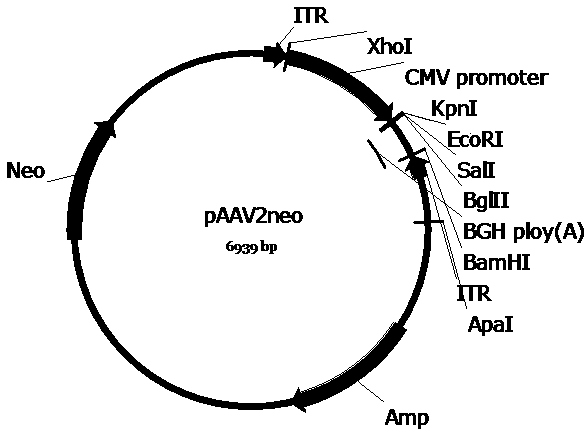
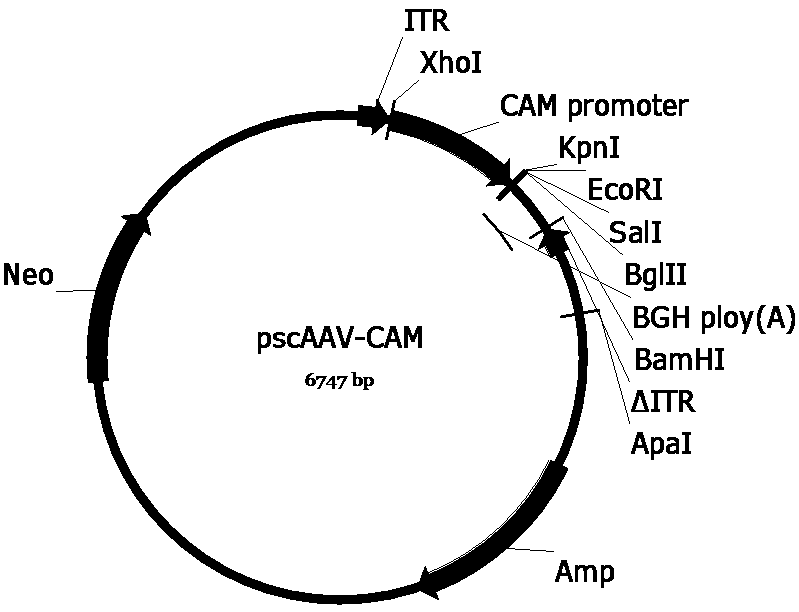
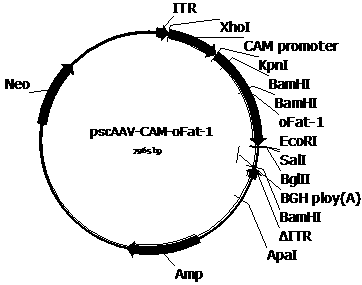
[0052] Example 1 Plasmid vector construction
[0053] In order to construct the pscAAV-CAM-OFat-1 and pscAAV-CAM-OFat-1-142T plasmids required for packaging recombinant AAV viruses, we first used the pAAV2neo preserved by the company as the basis and used the self-designed CAM promoter (SEQ IDNo. 1) Replace the CMV promoter in the pAAV2neo vector, and replace one of the pAAV2neo vectors with a mutated ITR sequence (named ΔITR) (SEQ ID No.2) that deletes the trs (terminal resolution site) and D sequences in the AAV2 ITR flanking ITR sequences to obtain the pscAAV-CAM vector. Next, the artificially synthesized OFat-1 (SEQ ID No.3) and OFat-1-142T (SEQ ID No.4) sequences were respectively cloned into the KpnI and EcoRI and KpnI and BglII restriction sites of the pscAAV-CAM vector Between, pscAAV-CAM-OFat-1 and pscAAV-CAM-OFat-1-142T vectors were obtained.
[0054] (1) Construction of pscAAV-CAM vector
[0055] The human cytomegalovirus early gene enhancer sequence, the chicken...
Embodiment 2
[0061] Example 2 Preparation and assay of recombinant AAV virus
[0062] References [102] , using the three-plasmid packaging system to package and purify the recombinant AAV virus. Briefly, the AAV vector plasmid (pscAAV-CAM-OFat-1 or pscAAV-CAM-OFat-1-142T), the helper plasmid (pHelper), and the AAV Rep and Cap protein expression plasmids (pAAV-R2C1, pAAV-R2C8 or pAAV -R2C9) mixed according to the molar ratio of 1:1:1, transfect HEK293 cells by calcium phosphate method, after 48 hours of transfection, harvest the cells and culture supernatant, and use cesium chloride density gradient centrifugation to separate and purify the recombinant AAV virus . Packaged and purified to obtain scAAV1-CAM-OFat-1, scAAV1-CAM-OFat-1-142T, scAAV8-CAM-OFat-1, scAAV8-CAM-OFat-1-142T, scAAV9-CAM-OFat-1 and scAAV9-CAM - 6 kinds of recombinant viruses such as OFat-1-142T.
[0063] Quantitative PCR method was used to measure the genome titer of the prepared AAV virus. The specific process is a...
Embodiment 3
[0068] Example 3 Intravenous administration for the treatment of type 1 diabetes
[0069] 70 8-week-old female NOD / LtJ (hereafter abbreviated as NOD) mice were purchased from Beijing Huafukang Biotechnology Co., Ltd., cultured under SPF conditions until 12 weeks, blood was collected from the tail vein, and a blood glucose meter (Accu-Chek , Roche) measured the non-fasting blood glucose concentration of each mouse, and regarded the mice with non-fasting blood glucose concentration higher than 13mM as type 1 diabetic mice, and obtained 51 mice in total. 40 of them were randomly divided into 5 groups with 8 mice in each group. Among the 5 groups of mice, 4 groups of mice were injected with scAAV9-CAM-OFat-1, scAAV9-CAM-OFat-1-142T, scAAV8-CAM-OFat-1 or scAAV8-CAM-OFat-1- 142T recombinant virus, the injection dose is 2×10 10 vg / only, and the remaining 1 group of mice were used as controls injected with scAAV9-CAM-OFat-1 or scAAV9-CAM-OFat-1-142T and mice injected with scAAV8-CAM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com