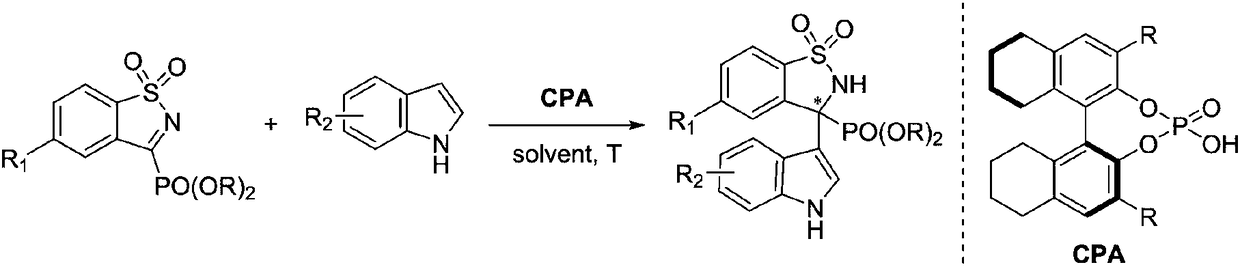
Method for synthesizing chiral aminophosphonates through organocatalytic Friedel-Crafts reactions
A technology of amino phosphonate and Friedel-Crafts reaction, applied in organic chemistry methods, organic chemistry, chemical instruments and methods, etc., can solve problems such as abundant nucleophilic reagents, achieve simple and practical reaction operation, complete reaction, and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
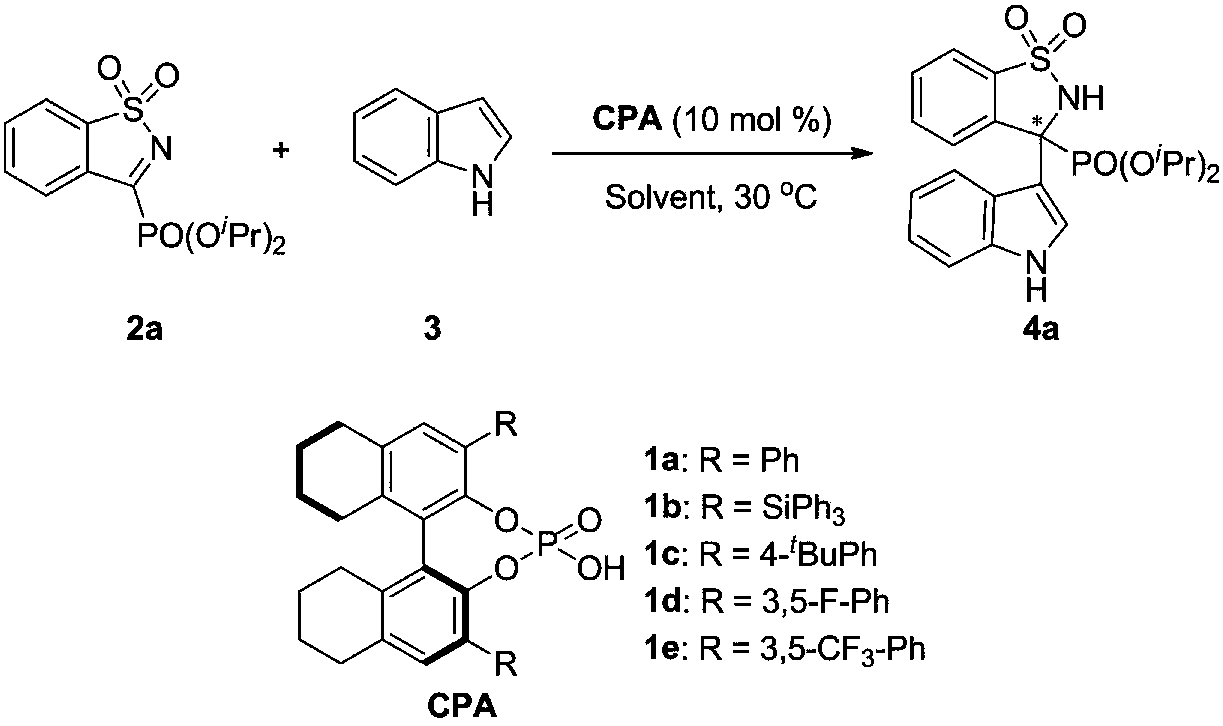
[0024] Embodiment 1: optimization of conditions
[0025] In the air, add five-membered cyclic imine phosphonate 2a (33mg, 0.1mmol), chiral phosphoric acid (10mol% of the amount of substrate in formula 1) and organic solvent (2mL) to the reaction flask successively, stir at room temperature for ten minutes, then added indole 3 (35mg, 0.3mmol), reacted at 30°C, and monitored the progress of the reaction by TLC. After the reaction, the pure product was obtained by direct column chromatography separation. The reaction formula and chiral phosphoric acid structure are as follows:
[0026]
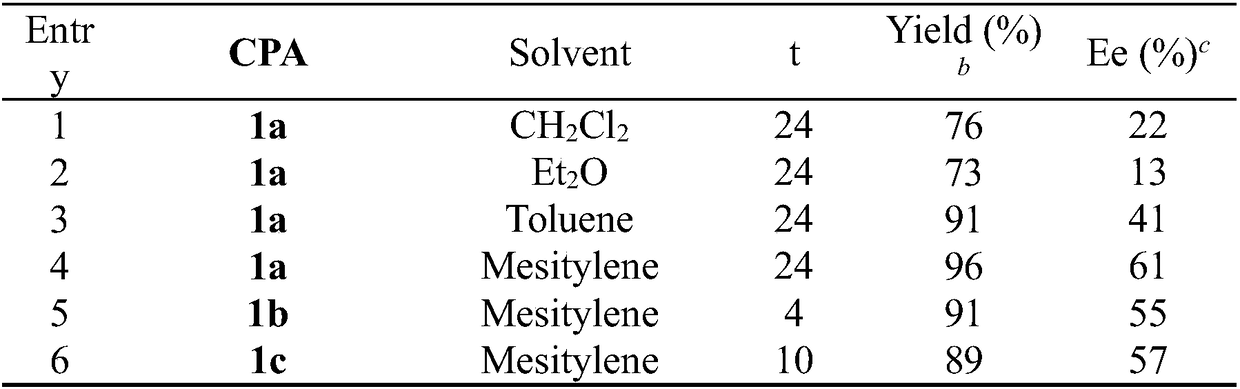
[0027] The yield is the separation yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, see Table 1 for details.
[0028] Table 1. Asymmetric addition reaction of imidophosphonate 2a with indolea
[0029]
[0030]
Embodiment 2
[0031] Example 2: Chiral Phosphoric Acid Catalyzed Friedel-Crafts Reaction Synthesis of Various Chiral Amino Phosphonates 4
[0032] In the air, add five-membered cyclic imine phosphonate 2 (0.1 mmol), chiral phosphoric acid (5 mol% of substrate consumption in formula 1) and organic solvent (2 mL) to the reaction flask successively, stir at room temperature for ten minutes, Then add indole 3 (35mg, 0.3mmol), react at 30°C, and monitor the progress of the reaction by TLC. After the reaction, the pure product was obtained by direct column chromatography separation. The reaction formula and chiral phosphoric acid structure are as follows:
[0033]
[0034] The yield is the separation yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography, see Table 2.
[0035] Table 2. Chiral phosphoric acid catalyzed Friedel-Crafts reaction for the synthesis of various chiral aminophosphonates4a
[0036]
[0037]
[0038] (R)-diisopropyl
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com