Method for synthesizing methacrylic anhydride
A technology of methacrylic anhydride and methacrylic acid, applied in the field of organic synthesis, can solve the problems of inability to overcome the intermittent production mode, unsuitable for industrial production, high production efficiency and energy consumption, and achieves high production efficiency, convenient for industrialization, and high product quality. Stable quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
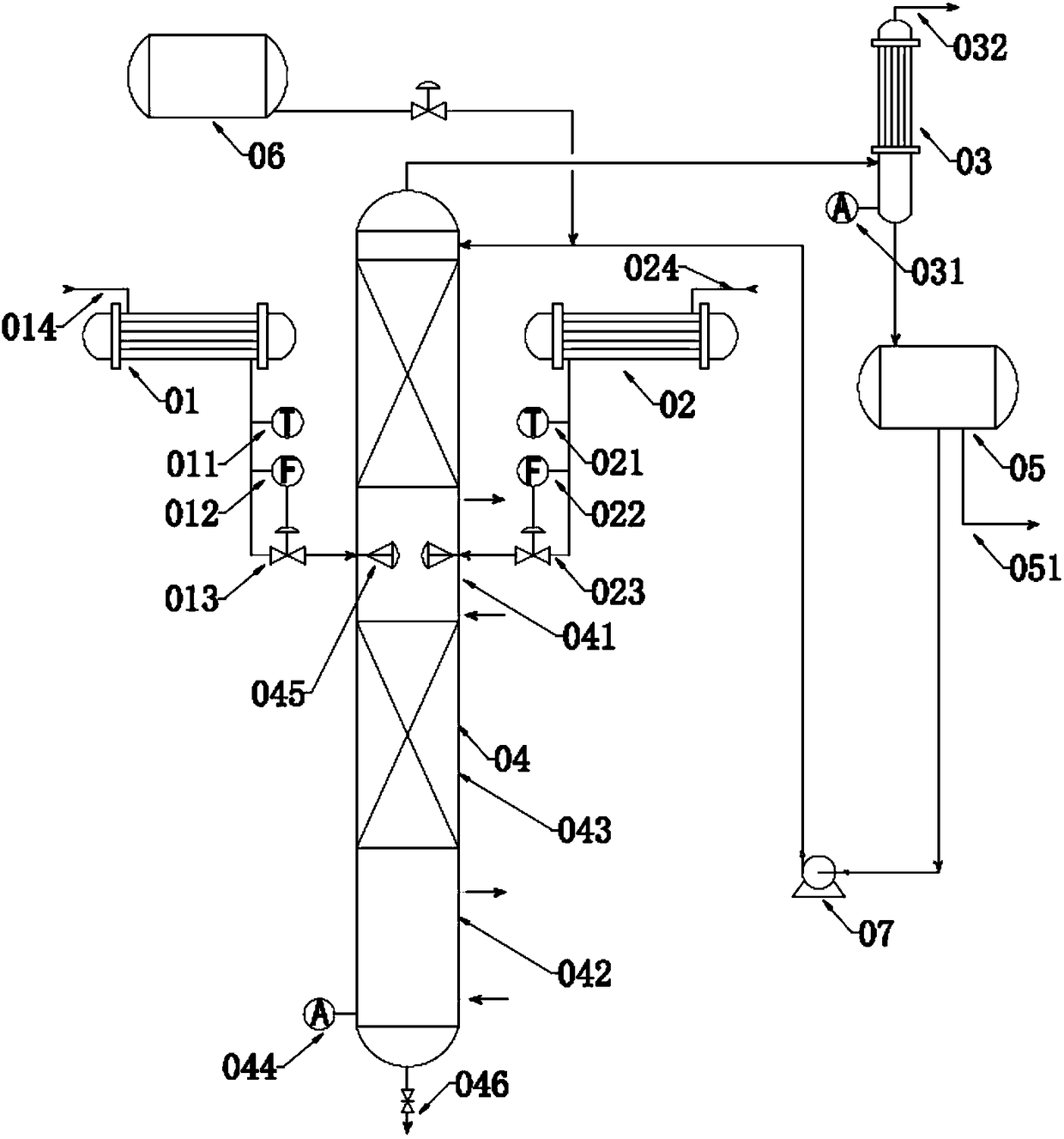
[0069] (1) Methacrylic acid enters the first heat exchanger (01) at the methacrylic acid inlet (014) for preheating. After preheating, the temperature is controlled by the temperature control device (011) to be 95 ° C, and enters the reactive distillation tower ( 04), keep a vacuum of -11KPa in the reactive distillation tower (04), and methacrylic acid is sucked in the reactive distillation tower (04) through the negative pressure in the reactive distillation tower (04), and is carried out through the atomizer (045). Atomization; At the same time, acetic anhydride enters the second heat exchanger (02) at the acetic anhydride inlet (024) for preheating. After preheating, the temperature is controlled by the temperature control device (021) to be 85 ° C, and enters the reactive distillation column (04 ), acetic anhydride is inhaled in the reactive distillation tower (04) by negative pressure in the reactive distillation tower, and is atomized through the atomizer (045) in the tow...
Embodiment 2
[0074](1) Methacrylic acid enters the first heat exchanger (01) at the methacrylic acid inlet (014) for preheating. After preheating, the temperature is controlled to 90°C by controlling the temperature control device (011), and enters the reactive distillation tower ( 04), keep a vacuum of -12KPa in the reactive distillation tower (04), methacrylic acid is sucked in the reactive distillation tower (04) through the negative pressure in the reactive distillation tower (04), and is carried out through the atomizer (045). Atomization; At the same time, acetic anhydride enters the second heat exchanger (02) at the acetic anhydride inlet (024) for preheating. After preheating, the temperature is controlled by the temperature control device (021) to be 90 ° C and enters the reactive distillation column (04 ), acetic anhydride is inhaled in the reactive distillation tower (04) by negative pressure in the reactive distillation tower, and is atomized through the atomizer (045) in the to...
Embodiment 3
[0079] (1) Methacrylic acid enters the first heat exchanger (01) at the methacrylic acid inlet (014) for preheating. After preheating, the temperature is controlled to be 100°C by controlling the temperature control device (011), and enters the reactive distillation tower ( 04), the vacuum in the reactive distillation tower (04) is maintained at -10KPa, and methacrylic acid is sucked into the reactive distillation tower (04) through the negative pressure in the reactive distillation tower (04), and is carried out through the atomizer (045). Atomization; At the same time, acetic anhydride enters the second heat exchanger (02) at the acetic anhydride inlet (024) for preheating. After preheating, the temperature is controlled by the temperature control device (021) to be 80 ° C and enters the reactive distillation column (04 ), acetic anhydride is inhaled in the reactive distillation tower (04) by negative pressure in the reactive distillation tower, and is atomized through the at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com