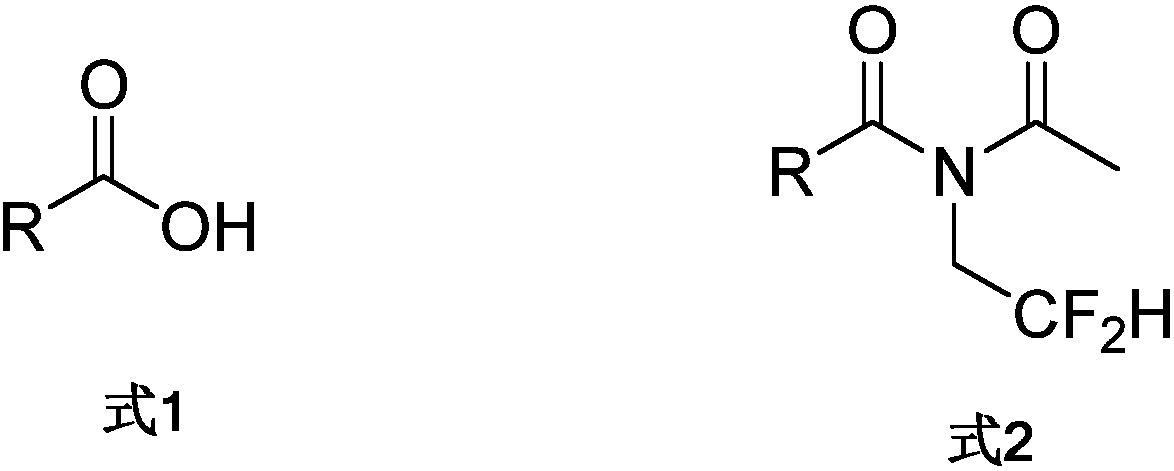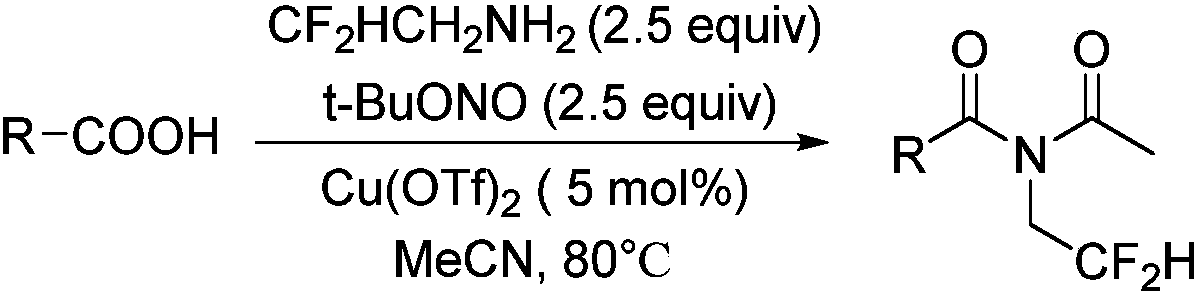N-acetyl-(N-difluoroethyl) amide compound and preparation method thereof
A technology of amide compounds and difluoroethyl, which is applied in the field of preparation of N-acetyl-amide compounds, can solve the problems of less by-products in product yield, cumbersome preparation process, harsh reaction conditions, etc., and achieve less side reactions, The process is easy to operate and the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
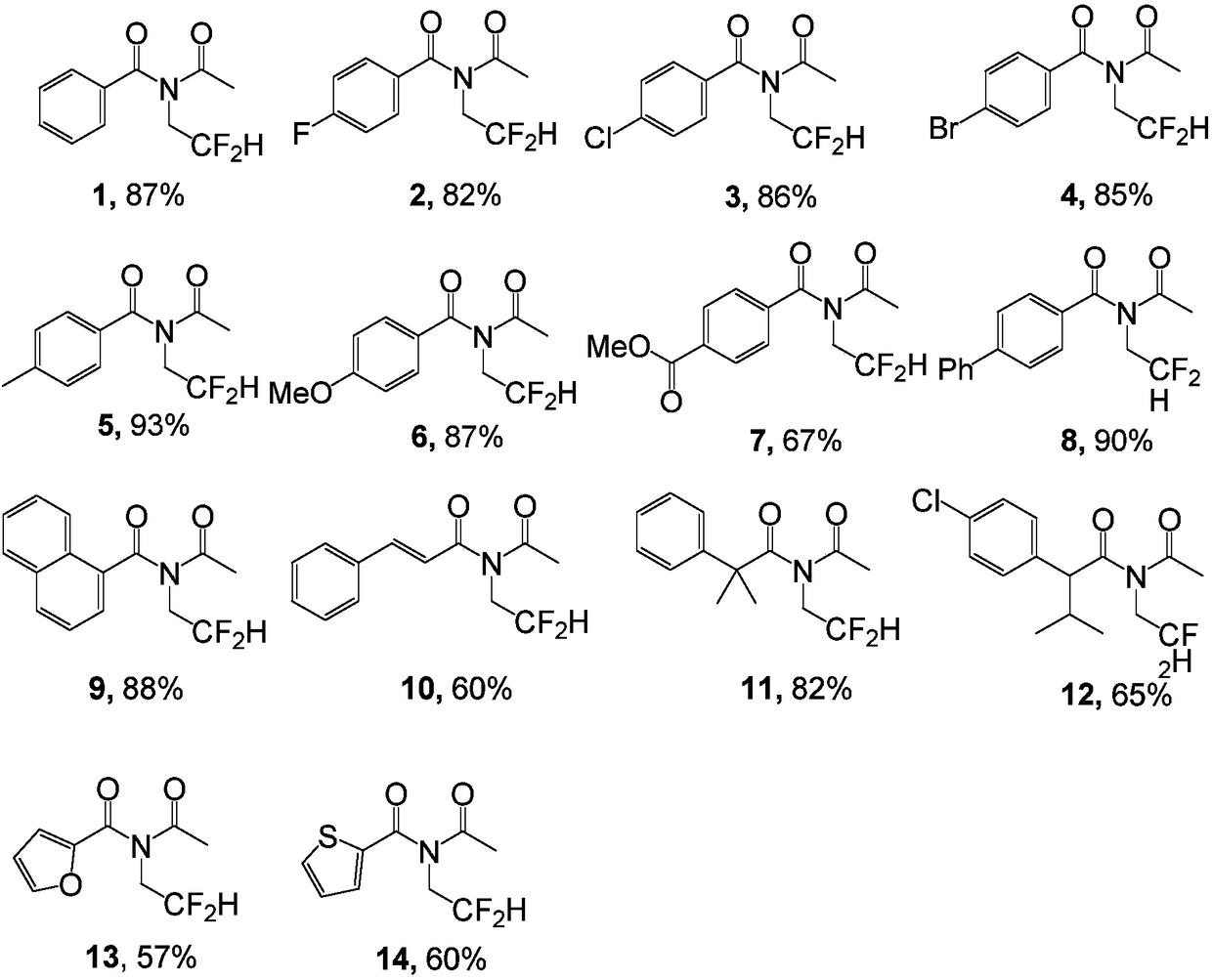
Embodiment 1~14
[0031] The following implementations 1-14 are prepared according to the following synthetic route, and compounds 1-14 can be prepared:
[0032]
Embodiment 1
[0034] Benzoic acid (24.5mg, 0.2mmol, 1equiv) and Cu(OTf) 2 (3.6mg, 0.01mmol, 5mol%) was dissolved in MeCN (5ml); at room temperature, tert-butyl nitrite (62μL, 0.5mmol, 2.5equiv) and 2,2-difluoroethylamine (38μL, 0.5mmol , 2.5 equiv) stirring, the temperature was raised to 65° C. and the reaction was continued for 12 hours. After the reaction was complete, spin-dried under reduced pressure, and separated by silica gel flash column chromatography to obtain the target compound 1 (40 mg, 0.173 mmol).
[0035] Substrate: Benzoic acid
[0036] product:
[0037] Compound 1: colorless oil (40mg, 87% yield); IR(KBr)ν max / cm -1 :3064,2977,1696,1671, 1598,1451,1339,1221,1117,1019,855,703; 1 H NMR (400MHz, CDCl 3 )δ7.64 (tt, J=5.7, 1.3 Hz, 2H), 7.62–7.58 (m, 1H), 7.53–7.47 (m, 2H), 6.08 (tt, J=56.8, 4.5Hz, 1H), 4.15 (td, J=13.5, 4.5Hz, 2H), 2.15(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ173.7, 173.3, 134.7, 133.1, 129.1, 128.7, 113.2 (t, J = 242.8Hz), 47.8 (t, J = 28.6Hz), 25.9; 1...
Embodiment 2
[0039] p-Fluorobenzoic acid (28mg, 0.2mmol, 1equiv) and Cu(OTf) 2 (3.6mg, 0.01mmol, 5mol%) was dissolved in MeCN (5ml), and at room temperature, tert-butyl nitrite (62μL, 0.5mmol, 2.5equiv) and 2,2-difluoroethylamine (38μL, 0.5mmol, 2.5 equiv) stirring, the temperature was raised to 80° C. and the reaction was continued for 20 hours. After the reaction was complete, spin-dried under reduced pressure, and separated by silica gel flash column chromatography to obtain the target compound 2 (42 mg, 0.164 mmol).
[0040] Substrate: p-Fluorobenzoic acid
[0041] product:
[0042] Compound 2: pale yellow oil (42mg, 82% yield); IR(KBr)ν max / cm -1 :3369,2922,2850,1707, 1676,1602,1507,1410,1337,1234,1219,1116,1026,986,852,770; 1 H NMR (400MHz, CDCl 3 ) δ7.81–7.61(m,2H),7.26–7.08(m,2H),6.07(tt,J=56.7,4.4Hz,1H),4.13(td,J=13.6,4.4Hz,2H),2.16 (s,3H); 13 C NMR (100MHz, CDCl 3 )δ 173.0, 172.6, 166.8, 164.2, 131.3 (d, J = 9.2 Hz), 130.8 (d, J = 3.3 Hz), 116.4 (d, J = 22.2 Hz), 113....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com