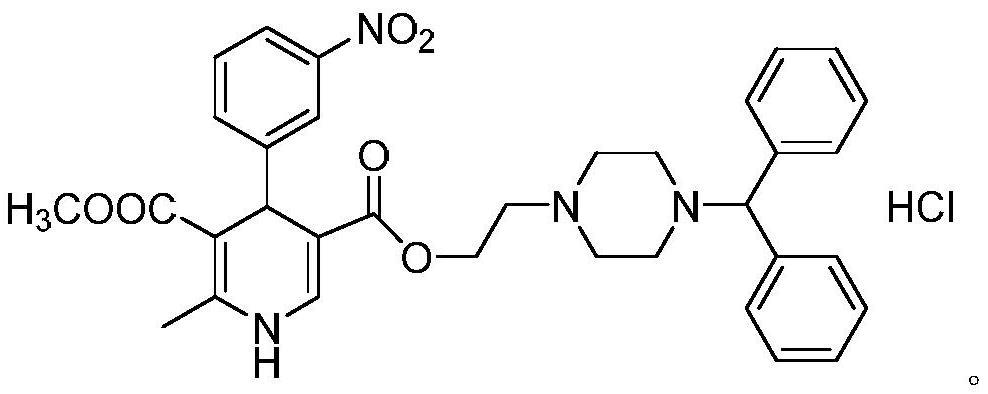
A kind of preparation technology of optically pure manidipine hydrochloride for treating hypertension
A manidipine hydrochloride, preparation technology, applied in the direction of organic chemistry, organic chemical methods, etc., can solve the problems of long time, low yield, high cost, etc., and achieve high ee value, simple operation, and short reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
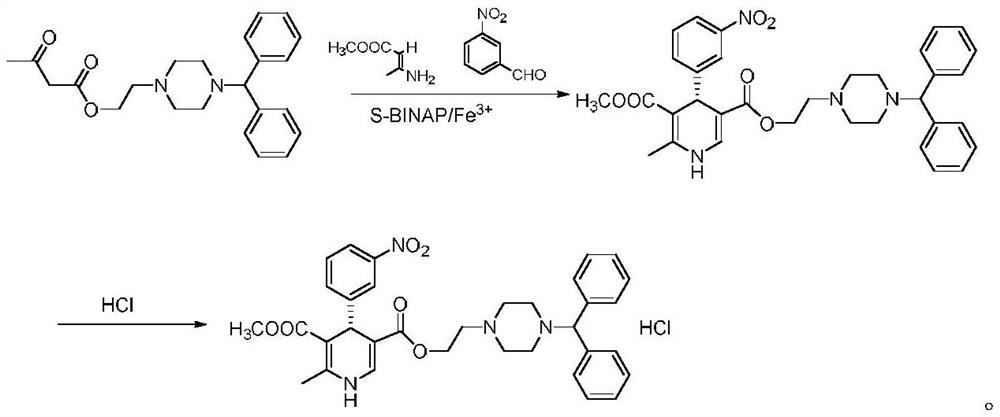
Examples
Embodiment 1
[0029] Preparation of (S)-manidipine
[0030] Sequentially add 4.56g (12mmol) of 2-(4-benzhydryl-1-piperazinyl)ethyl acetoacetate, 1.51g (10mmol) of m-nitrobenzaldehyde, and 1.21g of methyl 3-aminocrotonate (10.5mmol), (S)-BINAP 0.62g (1mmol), ferric chloride 0.4g (2.5mmol) and 50ml N,N-dimethylformamide were added to the reaction vessel, and the temperature was raised to 100°C for 2 hours to react. Concentrate under reduced pressure, recrystallize from n-hexane, and dry to obtain 4.68 g of (S)-manidipine, with a yield of 78.4% and an ee value of 99.37%.
Embodiment 2
[0032] Preparation of (S)-manidipine
[0033] Sequentially add 4g (10.5mmol) of 2-(4-benzhydryl-1-piperazinyl) ethyl acetoacetate, 1.51g (10mmol) of m-nitrobenzaldehyde, and 1.27g of methyl 3-aminocrotonate (11mmol), (S)-BINAP 0.93g (1.5mmol), ferric chloride 0.65g (4mmol) and 50ml N,N-dimethylformamide were added to the reaction vessel, the temperature was raised to 110°C and the reaction was carried out for 4 hours, and the pressure was reduced Concentrate, recrystallize from n-hexane, and dry to obtain 4.55 g of (S)-manidipine, with a yield of 76.2% and an ee value of 99.33%.
Embodiment 3
[0035] Preparation of (S)-manidipine
[0036] Sequentially add 4.19g (11mmol) of 2-(4-benzhydryl-1-piperazinyl)ethyl acetoacetate, 1.51g (10mmol) of m-nitrobenzaldehyde, and 1.38g of methyl 3-aminocrotonate (12mmol), (S)-BINAP 0.31g (0.5mmol), ferric bromide 0.59g (2mmol) and 50ml of toluene were added to the reaction vessel, heated to 90°C for 2 hours, concentrated under reduced pressure, recrystallized from n-hexane, After drying, 4.66 g of (S)-manidipine was obtained, with a yield of 78.1% and an ee value of 99.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com