Etilmicin derivative and preparation method thereof, pharmaceutical composition and application thereof
A technology of etimicin and derivatives is applied in the field of etimicin derivatives and preparation thereof, and achieves the effect of good resistance and significant resistance to drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
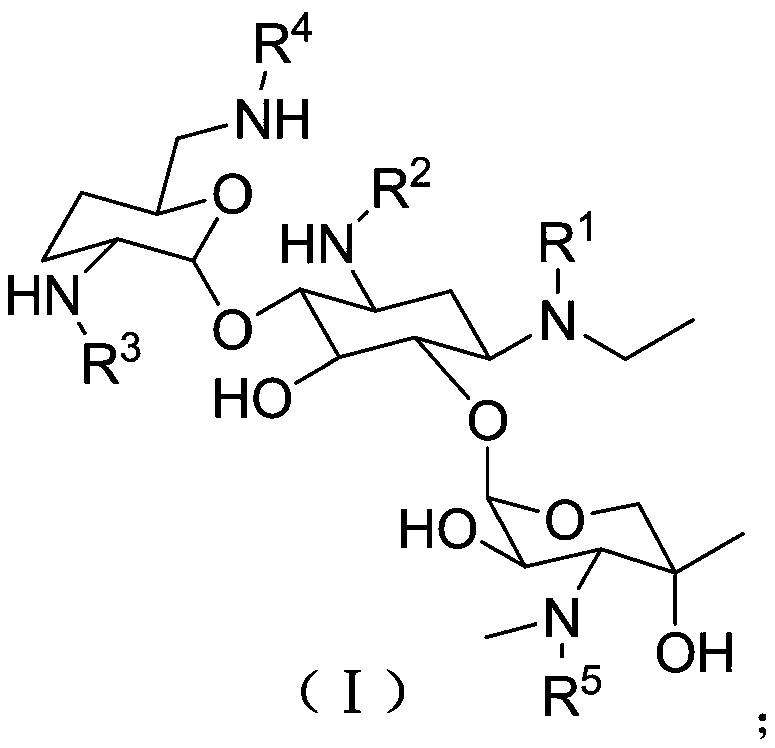
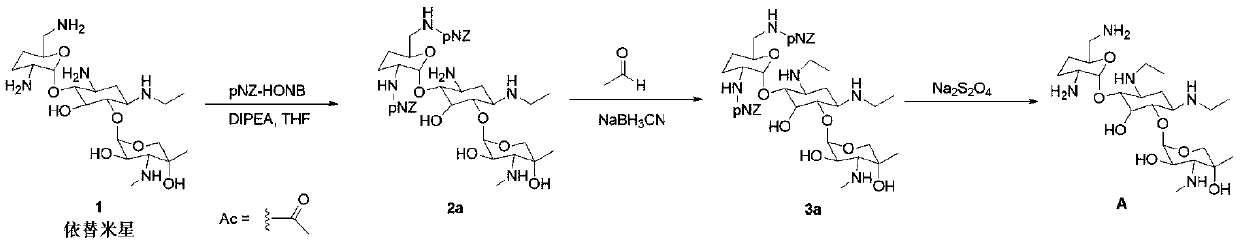
[0040] Preparation of derivative A substituted with etimicin amino group (preparation scheme a)
[0041] 1. Preparation of Compound 2a
[0042] Steps: Dissolve 5.10 g of etimicin in 150 mL of methanol, add 2.14 g of DIPEA, stir at room temperature for 5 minutes, then dissolve 3.81 g of HONB-pNZ in 50 mL of THF, and add dropwise to the reaction system at room temperature; After completion, the mixture was stirred overnight at room temperature, and the solvent was evaporated under reduced pressure, followed by silica gel column chromatography to obtain compound 2a; it was a white solid with a mass of 2.84 g and a yield of 31.9%. 1 H NMR (600MHz, CDCl 3 ):δ0.99(m,1H),1.11(s,3H),1.13(t,3H),1.48(m,1H),1.69(m,2H),1.96(m,1H),2.18(d, 1H),2.42(d,1H),2.53(m,1H),2.60(s,3H),2.61(m,1H),2.71(m,1H),2.85(m,1H),2.89(m,2H ),3.11(m,2H),3.22(t,2H),3.27(t,2H),3.38(m,1H),3.42(d,1H),3.45(s,2H),3.50(m,1H) ,3.54(m,1H),3.75(m,2H),3.99(m,1H),4.89(d,2H),5.14(m,2H),5.22(m,2H),7.47(d,2H), 7.53(d,2H),8.1...
Embodiment 2
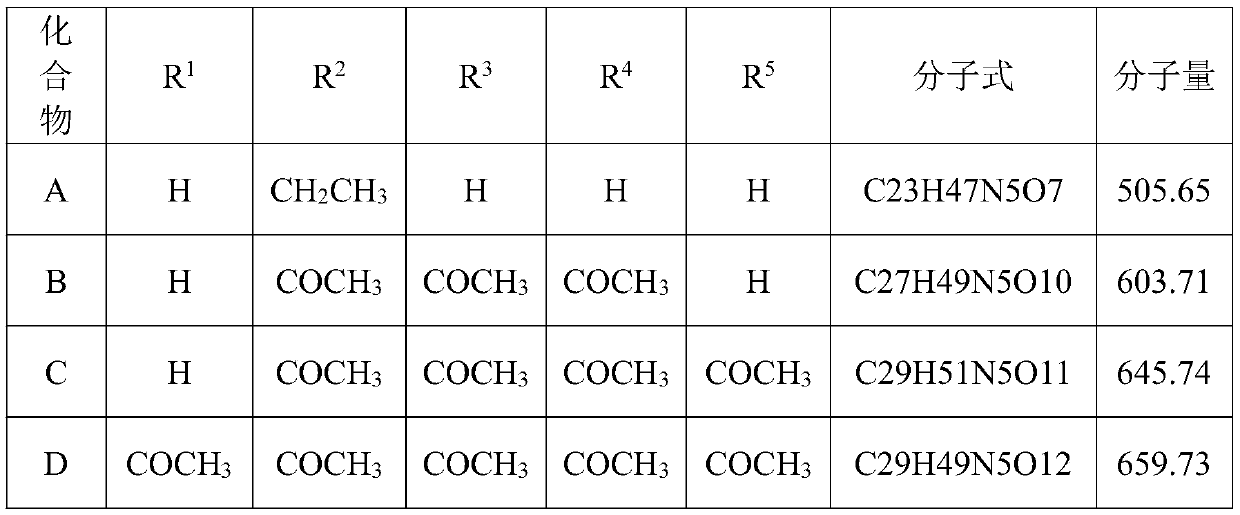
[0048] Preparation of derivatives B substituted with etimicin amino group (preparation scheme b)
[0049] Steps: Dissolve 2.31 g of etimicin in 50 mL of methanol, add 4.41 g of zinc acetate, and stir at room temperature for 10 minutes. After adding 2.04 g of acetic anhydride, the mixture was stirred overnight at room temperature. The solvent was distilled off under reduced pressure, and then separated by silica gel column chromatography to obtain Compound B with a mass of 1.79 g and a yield of 61.3%. 1 H NMR (600MHz,D 2 O): δ1.18(3H,s),1.32(m,1H),1.45(m,1H),1.66(m,3H),1.91(m,2H),1.94(m,6H),1.98(s ,3H),2.49(s,3H),2.59(d,1H),2.91(m,1H),3.19~3.30(m,4H),3.50(t,1H),3.59(t,1H),3.79( m,3H), 3.87(m,1H), 4.01(d,1H), 5.03(d,1H), 5.31(d,1H).ESIMS: Formula C 27 h 49 N 5 o 10 , found to be m / z 604.7 (M+H).
Embodiment 3
[0051] Preparation of derivative C substituted with etimicin amino group (preparation scheme c)
[0052] Steps: Dissolve 1.14 g of Compound B in 20 mL of methanol, add 1.04 g of acetic anhydride, and stir overnight at room temperature. The solvent was distilled off under reduced pressure, and then separated by silica gel column chromatography to obtain compound C with a mass of 1.10 g and a yield of 64.3%. 1 H NMR (600MHz,D 2 O): δ1.16(3H,s),1.46(m,1H),1.66(m,3H),1.89(m,1H),1.93(m,9H),1.98(s,3H),2.45(s ,3H),2.55(d,1H),3.24(m,3H),3.51(m,2H),3.66(m,2H),3.79(m,2H),3.88~3.97(m,2H),4.00( d,2H),5.02(d,1H),5.30(d,1H).ESIMS: Molecular formula C 29 h 51 N 5 o 11 , m / z 646.7 (M+H) was found.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com