Red light excited fluorescent dye as well as preparation method and application thereof
A technology for exciting fluorescence and dyes, applied in luminescent materials, azo dyes, organic dyes, etc., can solve the problem of difficulty in the synthesis of red light-excited fluorescent dyes, improve sensitivity and selectivity, enhance the fluorescence and photostability of organic molecules Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
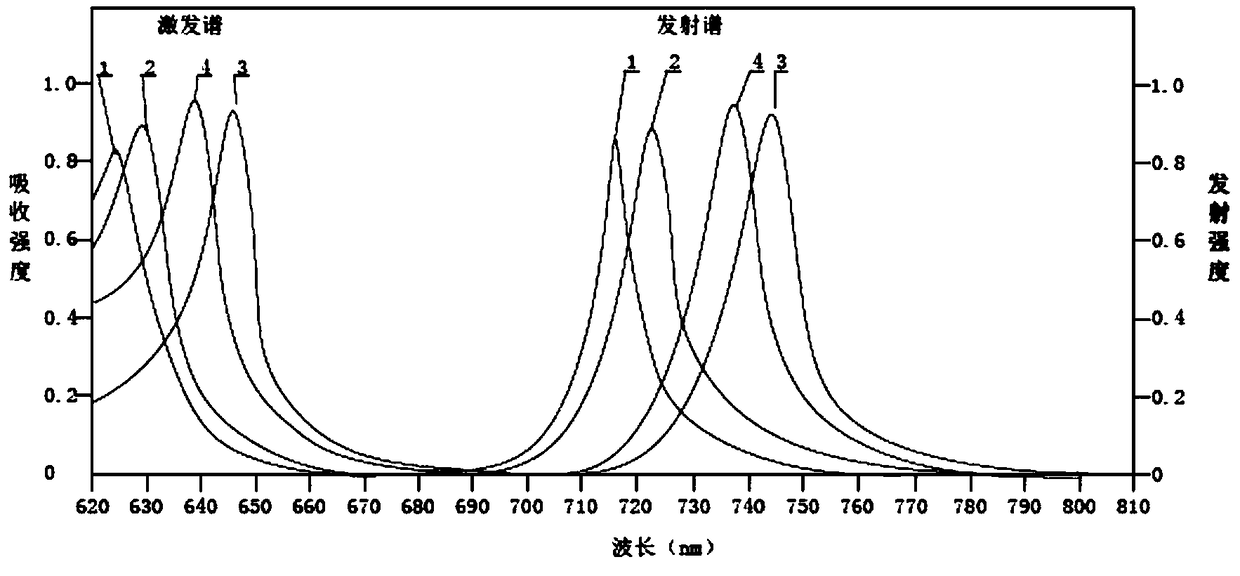
Image
Examples
Embodiment 1
[0034] The preparation method of the red light-excited fluorescent dye (II) provided in this example:
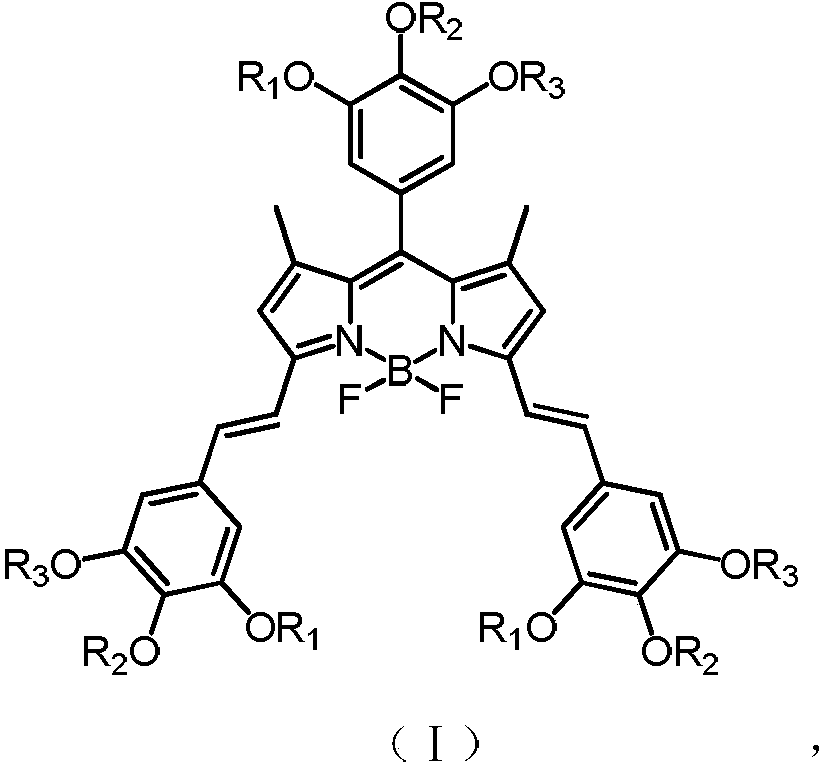
[0035] Has formula (II) structure:
[0036]
[0037] Its preparation method is as follows:
[0038]
[0039] (1) Preparation of intermediate 1-II
[0040] Under nitrogen protection, add 1.9g (0.01mol) of anhydrous CuI and 20ml THF to a 250ml reaction flask, cool to -55°C, add ClCH dropwise 2 CH=CHLi 1.23g (0.015m01), stirred for 35min, continued to cool to -80°C, added dropwise 2-chloro-4-methyl-2H-pyrrole 0.58g (0.005mol) in 60ml tetrahydrofuran solution, after the reaction, use 50ml of ethylene glycol dimethyl ether was diluted, washed with deionized water, and concentrated to obtain intermediate 1-II.
[0041] (2) Preparation of Intermediate 2-II
[0042] Add 1.2g (0.009mol) of anhydrous aluminum chloride and 24ml of dichloromethane into a 50ml reaction flask, place in an ice-salt bath, stir, cool down to 0°C, add 0.47g (0.003mol) of intermediate 1-II, Continue...
Embodiment 2
[0053] The preparation method of the red light-excited fluorescent dye (III) provided in this example:
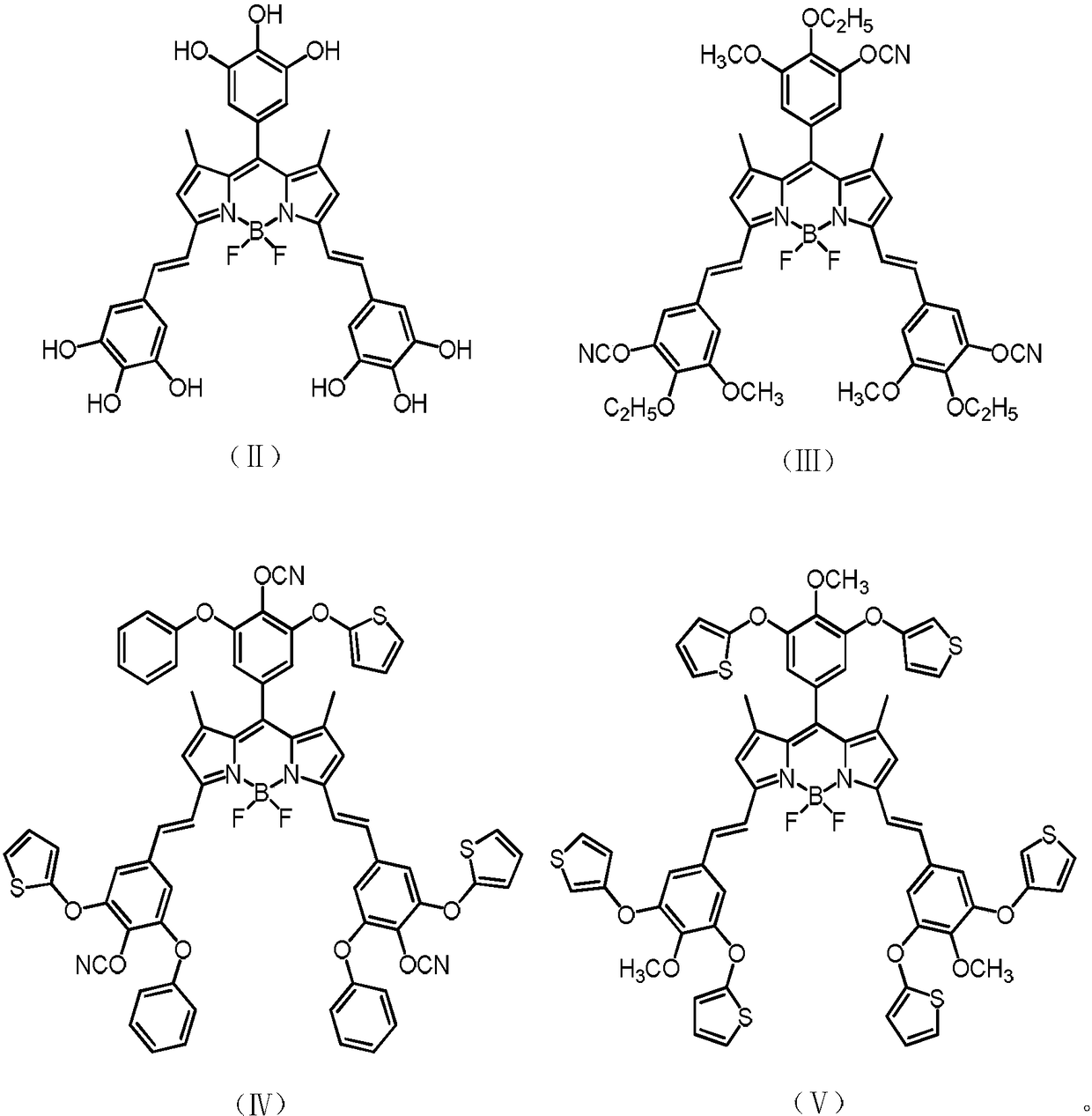
[0054] Has the structure of formula (III):
[0055]
[0056] Its preparation method is as follows:
[0057]
[0058] (1) Preparation of intermediate 1-III
[0059] Under nitrogen protection, add anhydrous CuI 5.7g (0.03mol) and 50ml THF to a 500ml reaction flask, cool to -50°C, add ClCH dropwise 2 CH=CHLi 4.1g (0.05mol), stirred for 40min, continued to cool to -78°C, added dropwise a solution of 1.7g (0.015mol) of 2-chloro-4-methyl-2H-pyrrole in 200ml tetrahydrofuran, after the reaction, use Diluted with 200ml of ethylene glycol dimethyl ether, washed with deionized water, and concentrated to obtain intermediate 1-III.
[0060] (2) Preparation of Intermediate 2-III
[0061] Add 3.3g (0.025mol) of anhydrous aluminum chloride and 66ml of dichloromethane into the reaction flask, place in an ice-salt bath, stir, cool down to -3°C, add 1.6g (0.01mol) of intermediate 1-...
Embodiment 3
[0072] The preparation method of the red light-excited fluorescent dye (IV) provided in this embodiment:
[0073] Has formula (IV) structure:
[0074]
[0075] Its preparation method is as follows:
[0076]
[0077] (1) Preparation of intermediate 1-IV
[0078] Under the protection of nitrogen, add anhydrous CuI 9.5g (0.05mol) and 100ml THF to a 1000ml reaction flask, cool to -50°C, add ClCH dropwise 2 CH=CHLi 6.2g (0.075mol), stirred for 30min, continued to cool to -75°C, added dropwise 2.9g (0.025mol) of 2-chloro-4-methyl-2H-pyrrole in 300ml tetrahydrofuran solution, after the reaction, use 250ml of ethylene glycol dimethyl ether was diluted, washed with deionized water, and concentrated to obtain intermediate 1-IV.
[0079] (2) Preparation of intermediate 2-IV
[0080] Add 5.3g (0.04mol) of anhydrous aluminum chloride and 130ml of dichloromethane into the reaction flask, place in an ice-salt bath, stir, cool down to -2°C, add 7.4g (0.02mol) of intermediate 2-IV, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com