Red light emission fluorescent dye as well as preparation method and application thereof
A fluorescent dye, red light technology, applied in luminescent materials, chemical instruments and methods, pharmaceutical formulations, etc., can solve the problems of easy photobleaching, low quantum yield, difficulty in synthesizing red light emitting fluorescent dyes, etc., and achieve photostability Good properties, simple synthesis process and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of the red light-emitting fluorescent dye (II) provided in this embodiment:
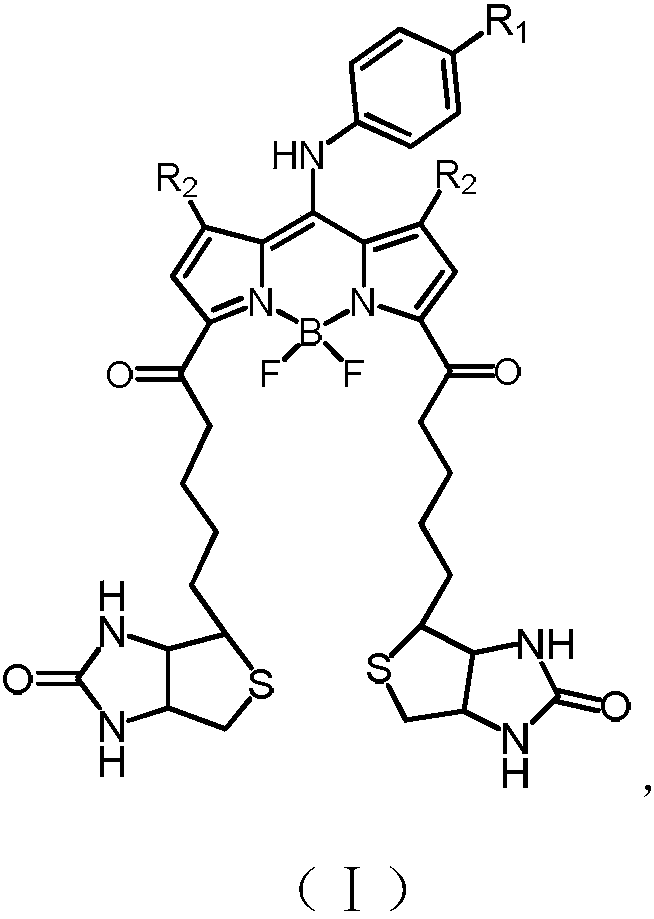
[0026] Has formula (II) structure:
[0027]
[0028] Its preparation method is as follows:
[0029]
[0030] S1. Take 10.0mL of chloroacetyl chloride and 30.0mL of 4-bromo-2-pyrrole carboxylic acid into a 500mL single-necked bottle, use 50mL of dichloromethane as a solvent, stir magnetically at 25°C to 28°C for 25min to 30min, and conduct thin layer analysis After the reaction of the raw material 4-bromo-2-pyrrole carboxylic acid was completed, the developer was ethyl acetate:petroleum ether=1:1, and directly evaporated to dryness at 30°C to obtain intermediate A of fluorescent dye (II).
[0031] S2. Add 200mL of boron trifluoride ether solution to the above-mentioned one-mouth bottle, slowly add 20mL of triethylamine dropwise, stir with magnetic force, control the temperature at 0-5°C, after the dropwise addition of triethylamine is completed, rise to 25°C-28°C f...
Embodiment 2
[0043] The preparation method of the red light-emitting fluorescent dye (III) provided in this embodiment:
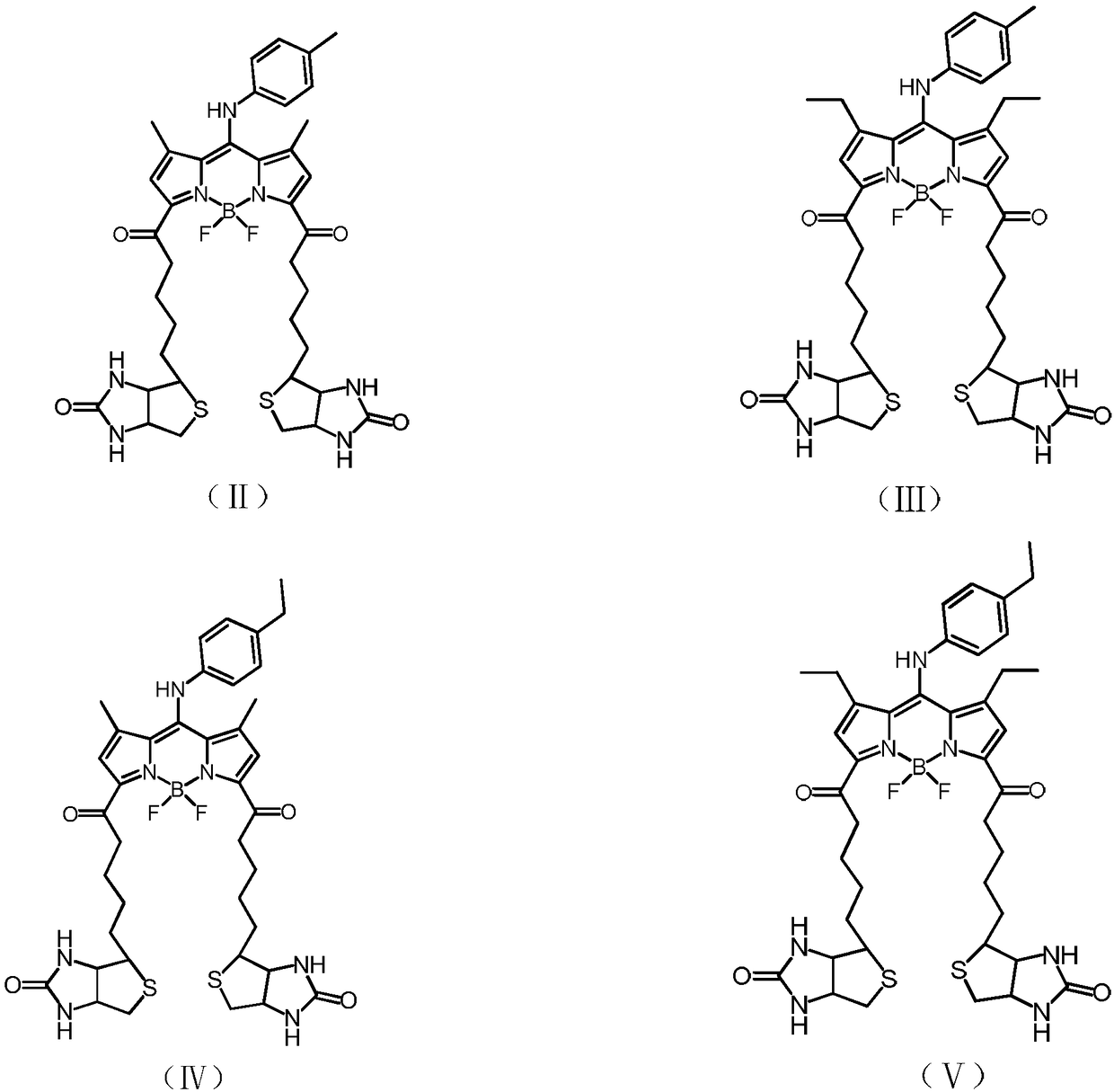
[0044] Has the structure of formula (III):
[0045]
[0046] Its preparation method is as follows:
[0047]
[0048] S1. Take 30.0mL of chloroacetyl chloride and 100.0mL of 4-bromo-2-pyrrole carboxylic acid into a 1000mL single-necked bottle, use 200mL of dichloromethane as solvent, stir magnetically at 25°C to 28°C for 25min to 30min, and conduct TLC analysis After the reaction of the raw material 4-bromo-2-pyrrole carboxylic acid was completed, the developer was ethyl acetate:petroleum ether=1:1, and directly evaporated to dryness at 30°C to obtain intermediate A of fluorescent dye (III).
[0049] S2. Add 200mL of boron trifluoride ether solution to the above-mentioned one-mouth bottle, slowly add 20mL of triethylamine dropwise, stir with magnetic force, control the temperature at 0-5°C, after the dropwise addition of triethylamine is completed, rise to 25°C-28°C...
Embodiment 3
[0061] The preparation method of the red light-emitting fluorescent dye (IV) provided in this embodiment:
[0062] Has formula (IV) structure:
[0063]
[0064] Its preparation method is as follows:
[0065]
[0066] S1. Take 15.0mL of chloroacetyl chloride and 50.0mL of 4-bromo-2-pyrrole carboxylic acid into a 500mL single-necked bottle, use 75mL of dichloromethane as solvent, stir magnetically at 25°C to 28°C for 25min to 30min, and conduct TLC analysis After the reaction of the raw material 4-bromo-2-pyrrole carboxylic acid was completed, the developer was ethyl acetate:petroleum ether=1:1, and directly evaporated to dryness at 30°C to obtain intermediate A of fluorescent dye (IV).
[0067] S2. Add 200mL of boron trifluoride ether solution to the above-mentioned one-mouth bottle, slowly add 20mL of triethylamine dropwise, stir with magnetic force, control the temperature at 0-5°C, after the dropwise addition of triethylamine is completed, rise to 25°C-28°C for reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com