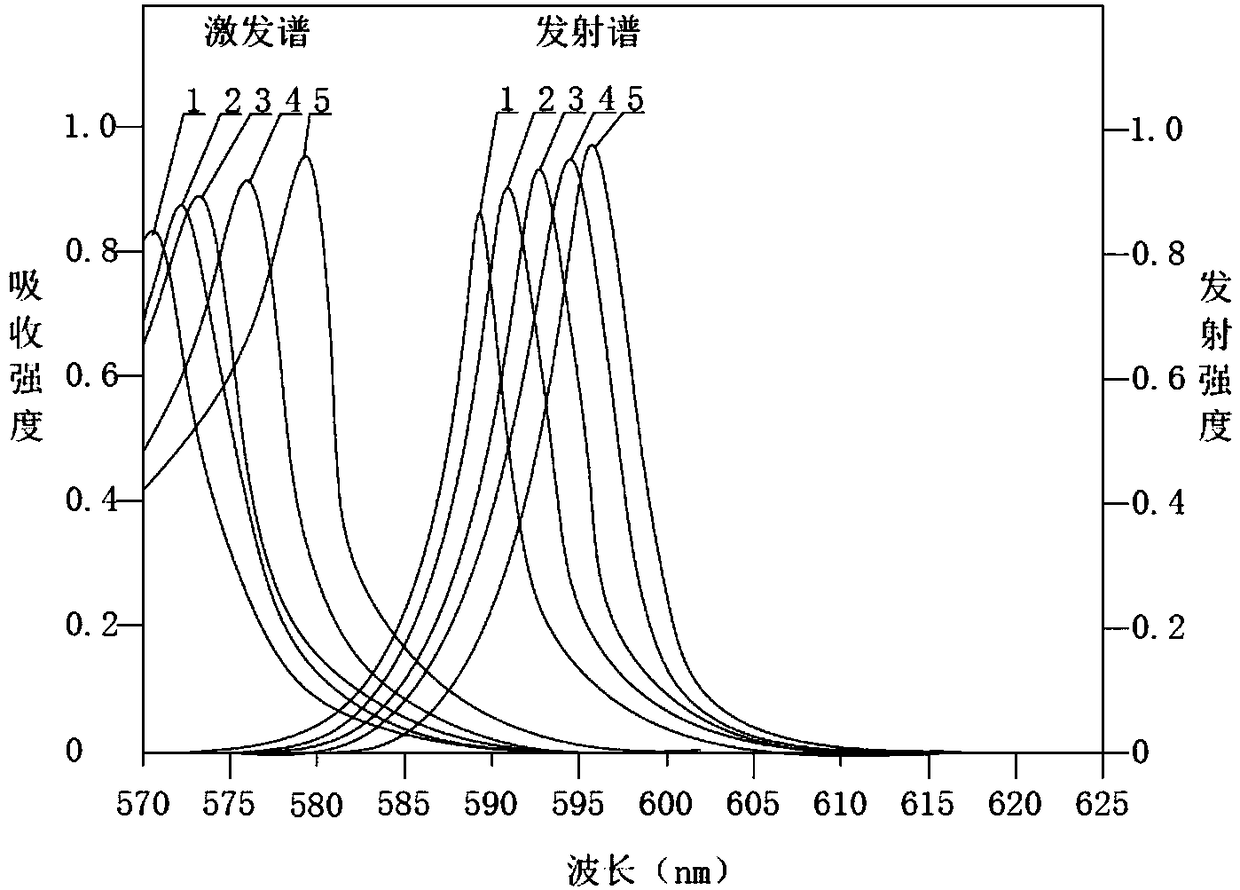
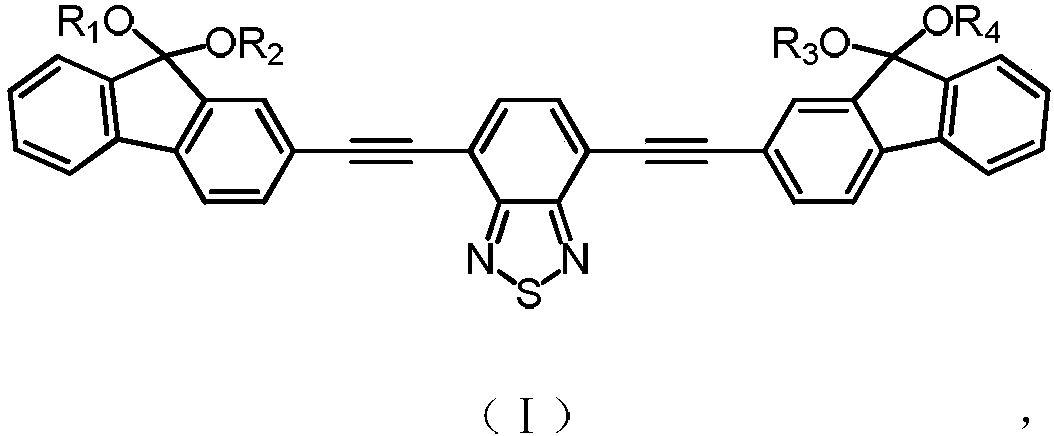
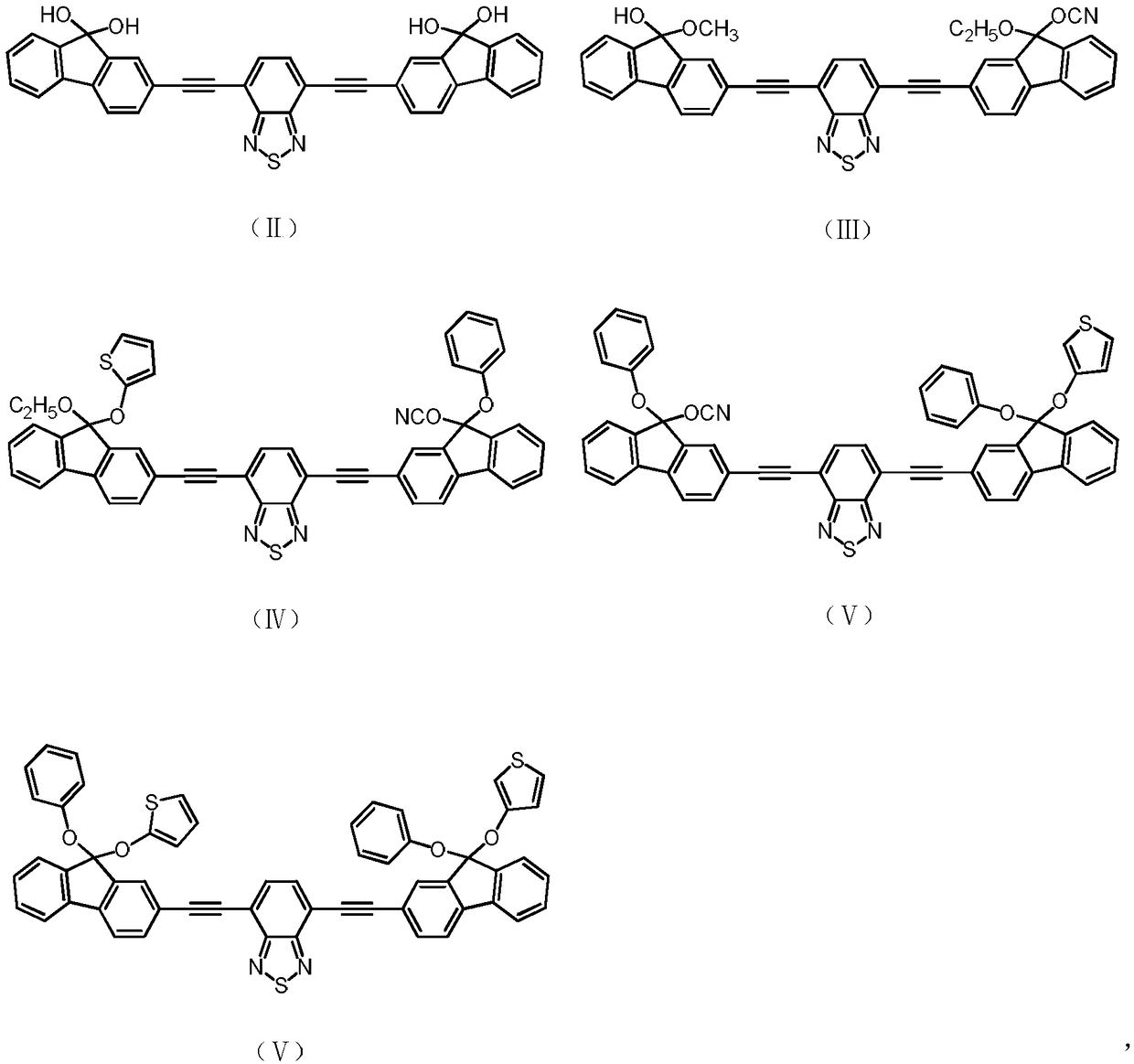
Yellow light excited fluorescent dye as well as preparation method and application thereof
A technology for exciting fluorescence and yellow light, applied in luminescent materials, azo dyes, organic dyes, etc., can solve the problem of difficulty in the synthesis of fluorescent dyes excited by yellow light, reduce the interference of self-absorption and self-fluorescence, and enhance the fluorescence of organic molecules , the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation method of the yellow light-excited fluorescent dye (II) provided in this embodiment:
[0036] Has formula (II) structure:
[0037]
[0038] Its preparation method is as follows:
[0039]
[0040] (1) Preparation of intermediate 1-II
[0041] Weigh 29.4g (0.1mol) of 4,7-dibromobenzo[c][1,2,5]thiadiazole, 1,2-bis(1,3,2-dioxaborolane-2- Base) 48.5g (0.25mol) of acetylene and 2.3g (0.002mol) of tetrakis (triphenylphosphine) palladium were added to a 1000ml three-necked flask, vacuumed and fed with nitrogen for 5min, 600ml of toluene was added, and 5.3g (0.05mol) of sodium carbonate was added. mol), heating to reflux, TLC detection of 4,7-dibromobenzo[c][1,2,5]thiadiazole, the reaction was completed, cooled to 20°C, extracted with dichloromethane, 100ml each time, and collected the organic layer , spin-dried, and passed through a column (MeOH:DCM=1:10) to obtain a solid, namely Intermediate 1-II.
[0042] (2) Preparation of intermediate 2-II
[004...
Embodiment 2
[0052] The preparation method of the yellow light-excited fluorescent dye (III) provided in this embodiment:
[0053] Has the structure of formula (III):
[0054]
[0055] Its preparation method is as follows:
[0056]
[0057] (1) Preparation of intermediate 1-III
[0058] Weigh 141.9 g (0.5 mol) of 4,7-dibromobenzo[c][1,2,5]thiadiazole, 1,2-bis(1,3,2-dioxaborolane-2- Base) 291.1g (1.5mol) of acetylene and 1.7g (0.015mol) of tetrakis (triphenylphosphine) palladium were added to a 10000ml three-neck flask, vacuumed and fed with nitrogen for 5min, 5000ml of toluene was added, and 26.5g (0.25mol) of sodium carbonate was added mol), heating to reflux, TLC detection of 4,7-dibromobenzo[c][1,2,5]thiadiazole, the reaction was completed, cooled to 20°C, extracted with dichloromethane, 1000ml each time, and collected the organic layer , spin-dried, and passed through a column (MeOH:DCM=1:10) to obtain a solid, namely Intermediate 1-III.
[0059] (2) Preparation of Intermedia...
Embodiment 3
[0069] The preparation method of the yellow light-excited fluorescent dye (IV) provided in this embodiment:
[0070] Has formula (IV) structure:
[0071]
[0072] Its preparation method is as follows:
[0073]
[0074] (1) Preparation of Intermediate 1-IV
[0075] Weigh 4,7-dibromobenzo[c][1,2,5]thiadiazole 116.7g (0.4mol), 1,2-bis(1,3,2-dioxaborolane-2- Base) 232.9g (1.2mol) of acetylene and 13.9g (0.012mol) of tetrakis (triphenylphosphine) palladium were added to a 5000ml three-necked flask, vacuumed and fed with nitrogen for 5min, 2400ml of toluene was added, and 21.2g (0.2mol) of sodium carbonate was added. mol), heating to reflux, TLC detection of 4,7-dibromobenzo[c][1,2,5]thiadiazole, the reaction was completed, cooled to 20°C, extracted with dichloromethane, 500ml each time, and collected the organic layer , spin-dried, and passed through a column (MeOH:DCM=1:10) to obtain a solid, namely Intermediate 1-IV.
[0076] (2) Preparation of intermediate 2-VI
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com