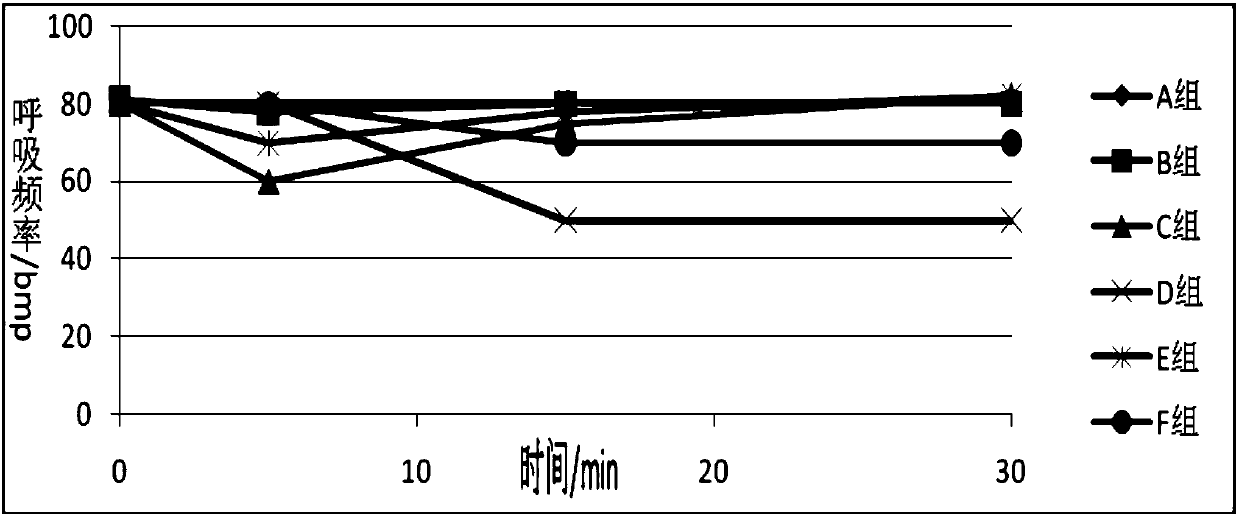
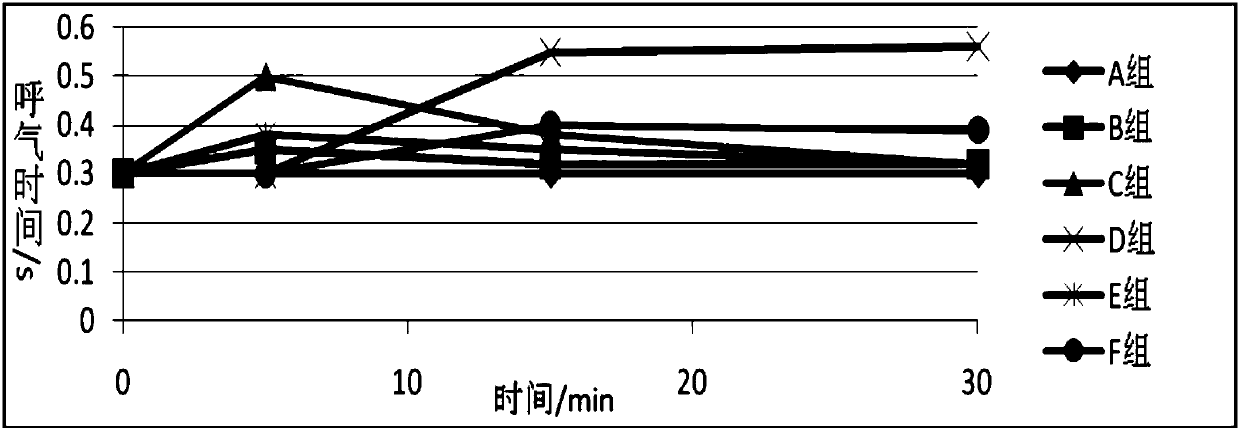
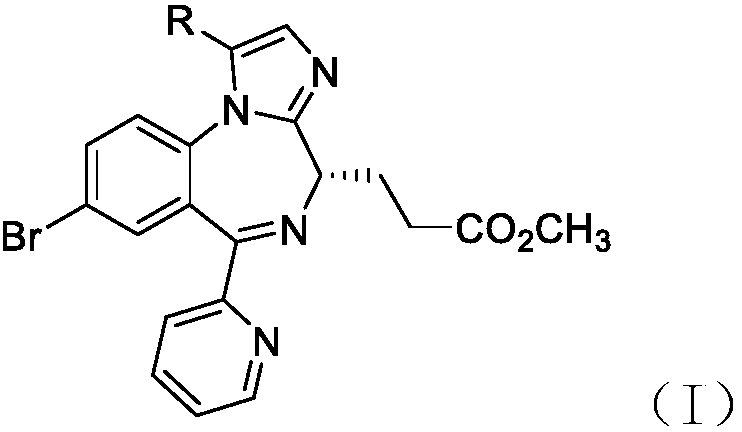
Anesthesia analgesia drug composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of easy occurrence of morning insomnia, daytime anxiety, low blood pressure and extrapyramidal symptoms, and achieve the effects of protecting physical and mental health, reducing the amount of auxiliary materials and improving stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: Preparation of the composition of narcotic analgesic drug
[0045] prescription:
[0046]
[0047] Preparation method: according to the feeding ratio, put dextran and glycine into about 90% of the prescription amount of water for injection to dissolve, and use a pH regulator to adjust the pH value of the above solution to 2.5-3.7. Then add formula (I) besylate and remifentanil hydrochloride while stirring, stir until completely dissolved, measure the pH of the solution to be 2.5 to 3.7, and then dilute to the full prescription amount with water for injection. Add 0.1% (w / v) medicinal charcoal according to the volume of the solution, and stir for 30 minutes. The medicinal solution is first filtered with a 0.45 μm microporous membrane, and then filtered with a 0.22 μm microporous membrane.
[0048] Freeze-drying process:
[0049] (1) Pre-freezing stage: After filling the filtered liquid, put it into the freeze-drying cabinet, start the freeze-drying ma...
Embodiment 2
[0053] Embodiment 2: Preparation of the composition of narcotic analgesic drug
[0054] prescription:
[0055]
[0056]
[0057] Preparation method: according to the feeding ratio, put lactose into about 90% of the prescription amount of water for injection to dissolve, and adjust the pH value of the above solution to 3.5-4.8 with a pH regulator. Then add formula (I) besylate and fentanyl citrate while stirring, stir until completely dissolved, measure the pH value of the solution to be 3.5 to 4.8, then dilute to the full amount of the prescription with water for injection. Add 0.01% (w / v) medicinal charcoal according to the volume of the solution, and stir for 40 minutes. The medicinal solution is first filtered with a 0.45 μm microporous membrane, and then filtered with a 0.22 μm microporous membrane.
[0058] Freeze-drying process:
[0059] (1) Pre-freezing stage: After filling the filtered liquid, put it into the freeze-drying cabinet, start the freeze-drying mach...
Embodiment 3
[0063] Embodiment 3: Preparation of the composition of narcotic analgesic drug
[0064] prescription:
[0065] components
Dosage
Compound benzenesulfonate shown in formula (I) (R is methyl, in base)
35g
Sufentanil citrate (calculated by base)
1.2g
7g
85g
pH regulator
Appropriate amount
Water for Injection
Dilute to 1000ml
[0066] Preparation method: according to the feeding ratio, put dextran and mannitol into about 90% of the prescription amount of water for injection to dissolve, and use a pH regulator to adjust the pH value of the above solution to 3.8-4.5. Then add formula (I) besylate and sufentanil citrate while stirring, stir until completely dissolved, measure the pH value of the solution to be 3.8 to 4.5, and then dilute to the full amount of the prescription with water for injection. Add 0.05% (w / v) medicinal charcoal according to the volume of the solution, and stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com